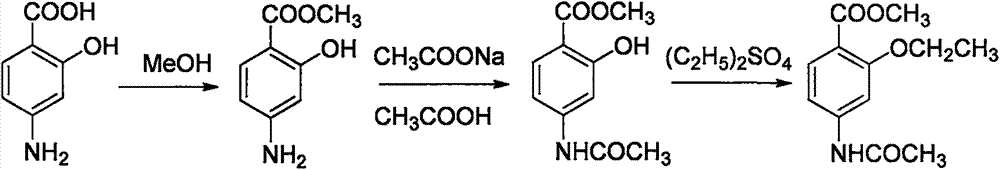
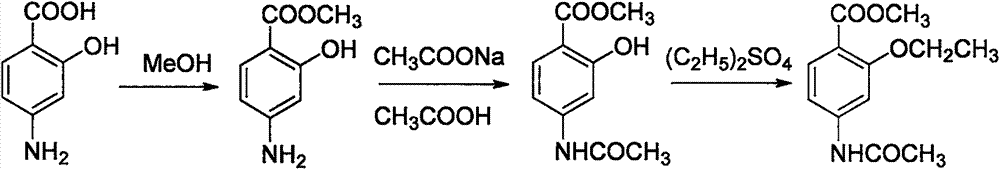
Preparation method of ethopabate
A technology of ethopabate and methyl acetylaminosalicylate, which is applied in the field of chemical drug synthesis, can solve the problems of not being green and environmentally friendly, and achieve the effects of environmental friendliness, high yield, and resource saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put (100.0g, 0.67mol) p-aminosalicylic acid and 5.0g p-toluenesulfonic acid in a 2L three-necked flask, add 500g methanol and stir evenly, raise the temperature to 65°C, and keep warm at 60-65°C for 4 hours. A reaction solution of methyl p-aminosalicylate was obtained.
[0020] Add (115.7g, 0.85mol) sodium acetate trihydrate to the reaction solution of methyl p-aminosalicylate, use 10% sodium carbonate or 10% acetic acid to control the pH range of the reaction solution to be 6.5-7.5, and control the temperature at 35- At 40°C, add 20.0 g of acetylase, strictly control the temperature and pH, react for 3 hours, and the reaction is complete. Methanol was recovered under reduced pressure, 500g of ethyl acetate was added to the mother liquor for extraction, liquid separation, drying and concentration were carried out, and then after cooling down and crystallization, suction filtration, and blast drying at 50°C, 196.7g of methyl p-acetylaminosalicylate was obtained. Yield 9...
Embodiment 2
[0023] Put (100.0g, 0.67mol) p-aminosalicylic acid and 8.0g p-toluenesulfonic acid in a 2L three-necked flask, add 800g methanol and stir evenly, raise the temperature to 65°C, and keep warm at 60-65°C for 3 hours. A reaction solution of methyl p-aminosalicylate was obtained.
[0024] Add (136.8g, 1.0mol) sodium acetate trihydrate to the reaction solution of methyl p-aminosalicylate, use 10% sodium carbonate or 10% acetic acid to control the pH range of the reaction solution to be 6.5-7.5, and control the temperature at 35- Add 40.0 g of acetylase at 40°C, strictly control the temperature and pH, react for 2 hours, and the reaction is complete. Methanol was recovered under reduced pressure, 500g of ethyl acetate was added to the mother liquor for extraction, liquid separation, drying and concentration were carried out, and then after cooling down and crystallization, suction filtration, and blast drying at 50°C, 199.2g of methyl p-acetylaminosalicylate was obtained. Yield 95....
Embodiment 3
[0027] Put (100.0g, 0.67mol) p-aminosalicylic acid and 10.0g p-toluenesulfonic acid in a 2L three-necked flask, add 600g methanol and stir evenly, raise the temperature to 65°C, keep warm at 60-65°C for 2 hours, A reaction solution of methyl p-aminosalicylate was obtained.
[0028] Add (115.7g, 0.85mol) sodium acetate trihydrate to the reaction solution of methyl p-aminosalicylate, use 10% sodium carbonate or 10% acetic acid to control the pH range of the reaction solution to be 6.5-7.5, and control the temperature at 35- 40°C, add 50.0g acetylase, strictly control the temperature and pH, react for 1 hour, and the reaction is complete. Methanol was recovered under reduced pressure, 500g of ethyl acetate was added to the mother liquor for extraction, liquid separation was carried out, drying and concentration was carried out, and then after cooling, crystallization and suction filtration, 195.9g of methyl para-acetamidosalicylate was obtained by air-drying at 50°C. Yield 93.6%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com