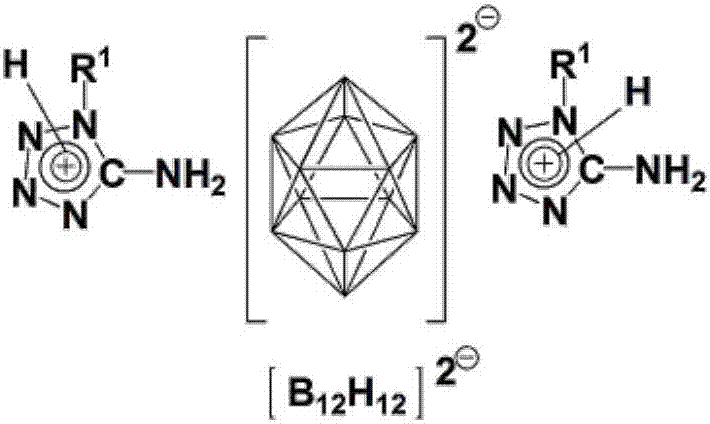
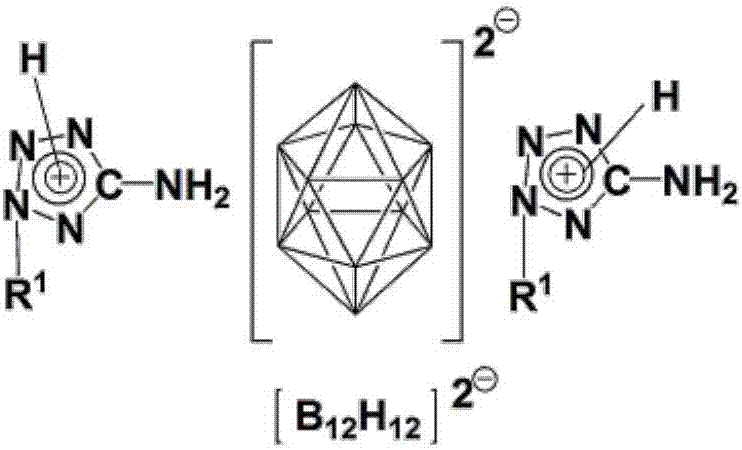
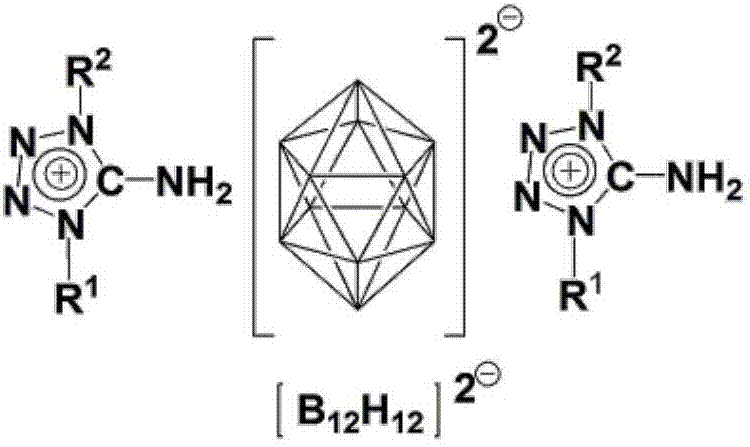
Bis(1-alkyl-5-aminotetrazolium)dodecahydrododecaborate or bis(2-alkyl-5-aminotetrazolium)dodecahydrododecaborate and bis(5-amino-1,4-dialkyltetrazolium)dodecahydrododecaborate or bis(5-amino-1,3-dialkyltetrazolium)dodecahydrododecaborate as well as preparation method thereof
A technology of dodecahydrododecaboron and aminotetrazolium salts, which is applied in the field of high-energy-density material preparation chemistry, can solve the problems of limitation, inconvenient performance testing and application research, complicated preparation and purification processes, etc., and achieves easy purification and processing The effect of convenience and simple procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 9. Suspend 9018g of 1-methyl-5-aminotetrazole in methanol, heat to reflux at 70°C, add excess concentrated hydrochloric acid, react for 2 hours, remove the solvent by suspension evaporation, and then recrystallize with water / methanol to obtain 1-methyl-5 -aminotetrazole hydrochloride; then 1-methyl-5-aminotetrazole hydrochloride and Ag 2 B 12 h 12 Dissolve or suspend in appropriate amount of distilled water respectively, react for half an hour, filter to remove solid matter, then concentrate and cool to crystallize to obtain bis(5-amino-1-methyltetrazole) dodecahydrododecaborate; at 70°C Dry under reduced pressure. 1 H NMR (DMSO-d 6 ,ppm,600MHz):δ8.36(s,6H,2NH 3 ),3.71(s,6H,2NCH 3 ), 1.3-0.4 (platform, 12H, B 12 h 12 ). 13 C NMR (DMSO-d 6 ,ppm,600MHz):δ155.16(ring C),32.26(N-C). 11 BNMR (DMSO-d 6 ,ppm,600MHz):δ-15.5.IR(cm -1 ):ν3358,3302,3246,3172,2459,1681,1027,712,684.ESI-MS(m / z):100([M C2H6N5 ] + ),70.95([M B12H12 / 2] - )
Embodiment 2
[0038] 0.66g K 2 B 12 h 12 and 1.45g of 5-amino-1,4-dimethyltetrazolium iodide were dissolved in deionized water and methanol respectively, and the two were mixed and reacted for 1.5h, then heated at 65°C for half an hour, concentrated and crystallized to obtain twelve Bis(5-amino-1,4-dimethyltetrazolium) hydrogen dodecaborate salt white crystal; dried under reduced pressure at 80°C. 1 H NMR (DMSO-d 6 ,ppm,600MHz):δ9.09(s,4H,2NH 2 ),3.87(s,12H,4NCH3 ), 1.3-0.4 (platform, 12H, B 12 h 12 ). 13 C NMR (DMSO-d 6 ,ppm,600MHz):δ149.0(ring C),34.5(N-C). 11 B NMR (DMSO-d 6 ,ppm,600MHz):δ-15.5.IR(cm -1 ):ν3341,3294,3248,3116,2487,1681,1606,1532,1437,1185,1046,771,716.ESI-MS(m / z):114([M C3H8N5 ] + ),71.35([M B12H12 / 2] - )
Embodiment 3
[0040] (Et 3 NH) 2 B 12 h 12 and 5 amino-1,4-dimethyltetrazolium iodide were mixed in water and acetonitrile at a molar ratio of 1:2 and reacted at reflux for 2 hours, the solvent was removed under reduced pressure, and the residue was recrystallized from water-methanol to obtain twelve Bis(5-amino-1,4-dimethyltetrazolium) hydrogen dodecaborate salt white crystal. Test data is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com