A kind of highly enantioselective C-5 α-stereocenter 4-nitroisoxazole alcohol compound, its preparation method and application
A nitroisoxazolol and enantioselectivity technology, applied in the field of drug synthesis, can solve problems such as not being reported, and achieve the effects of low reaction cost, reasonable reaction path and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The structural formula of compound 3a is as follows:
[0049]
[0050] Product name: (R)-2-(3-methyl-4-nitroisoxazole-5)-propan-1 alcohol.
[0051] The synthetic route of compound 3a is as follows:
[0052]
[0053] The synthetic steps of compound 3a are as follows:
[0054] (1) 3-methyl-5-ethyl-4-nitroisoxazole 1a (0.1mmol, 1.0equiv.), catalyst A (0.01mmol, 0.1equiv.), sodium acetate (0.1mmol, 1.0equiv. )and Molecular sieves (50 mg) were dissolved in 1.0 mL of cyclopentyl methyl ether, and stirred at room temperature for 15 minutes;
[0055] (2) Add paraformaldehyde 2a (0.3mmol, 3.0equiv.), and continue the reaction at room temperature until the raw material 1a disappears completely (about 70h) as monitored by TLC;
[0056] (3) Spin to dry the solvent and separate by column chromatography (eluent: petroleum ether and ethyl acetate volume ratio from 10:1 to 3:1) to obtain colorless oily liquid 3a (85% yield).
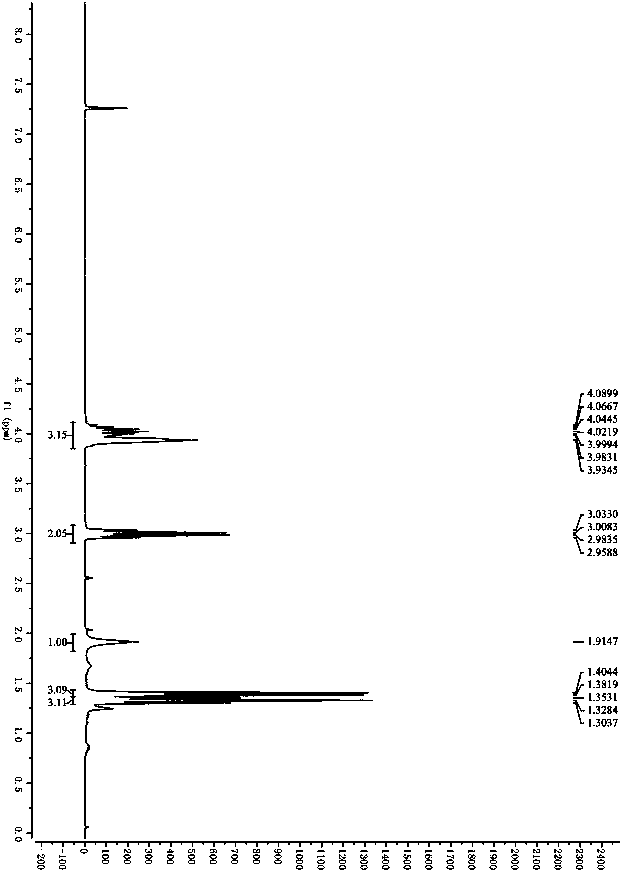
[0057] Colorless oily liquid, 97%ee, 1 H NMR...
Embodiment 2
[0060]
[0061] Product name: Product name: (R)-2-(3-ethyl-4-nitroisoxazole-5)-propan-1 alcohol.
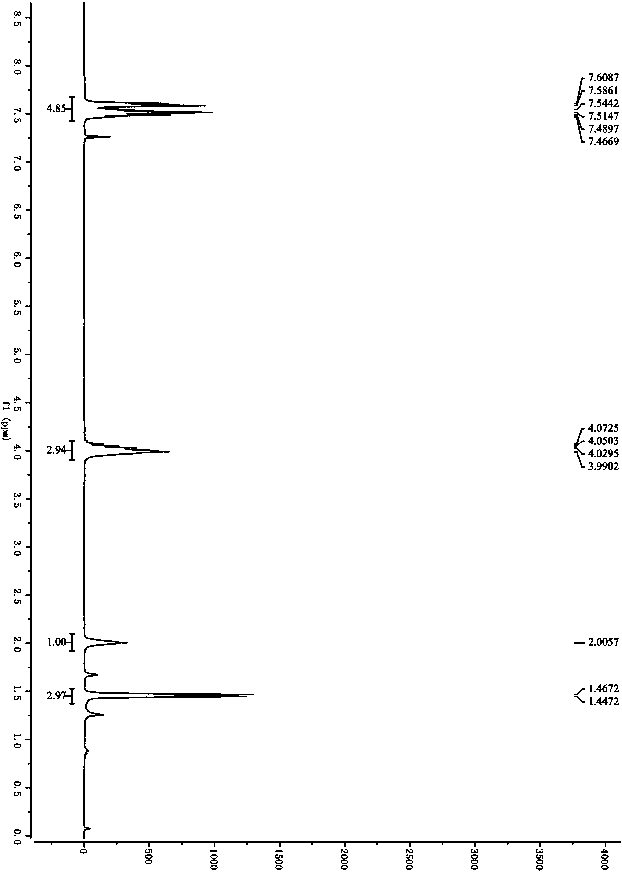
[0062] In step (1), replace 3-methyl, 5-ethyl, 4-nitroisoxazole 1a with 3,5-ethyl-4-nitroisoxazole 1b, and refer to the implementation of other experimental steps and purification methods Carried out in Example 1; Pale yellow oil, 97%ee, 86%yield, 1 H NMR (300MHz, CDCl 3 )δ4.11-3.85 (m, 3H), 3.00 (q, J = 7.4Hz, 2H), 1.91 (s, 1H), 1.39 (d, J = 6.8Hz, 3H), 1.33 (t, J = 7.4 Hz,3H), specifically as image 3 shown; 13 C NMR (75MHz, CDCl 3 )δ176.3, 160.1, 129.8, 64.9, 36.2, 19.8, 13.8, 11.1; HRMS (ESI) m / z 201.0878 (M+H + ), calc.for C 8 h 13 N 2 o 4 201.0875.
[0063] The ee value of the compound was analyzed by high performance liquid chromatography (HPLC), CHIRALPAK IE (4.6mm×250mmi.d.), n-hexane / isopropanol=80 / 20 (V / V), flow rate 1.0mL / min, 25°C, 254nm, t R = 7.8 min (small peak) and 8.3 min (large peak).
Embodiment 3
[0065] Product name: (R)-2-(3-phenyl-4-nitroisoxazole-5)-propan-1 alcohol.
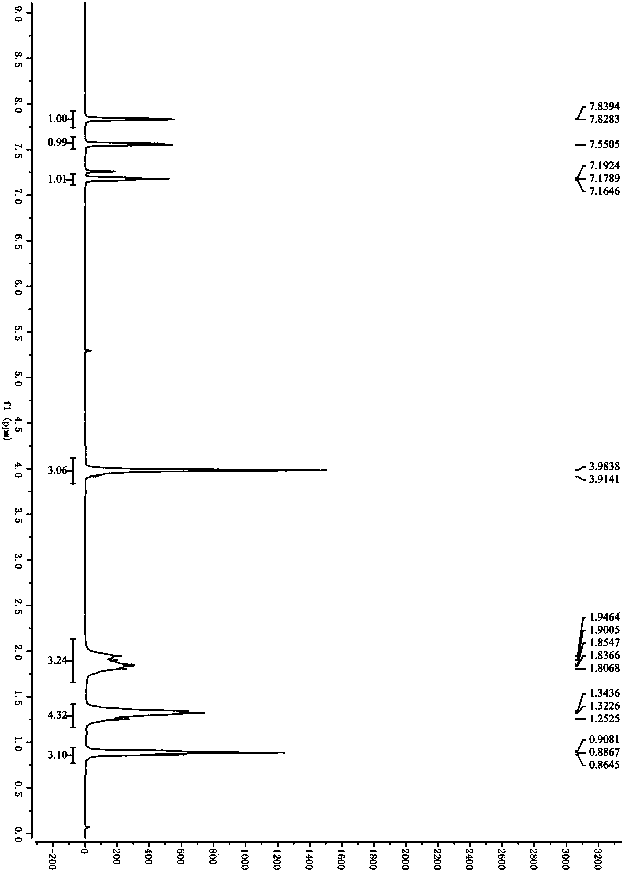
[0066] In step (1), replace 3-methyl, 5-ethyl, 4-nitroisoxazole 1a with 3-phenyl-5-ethyl-4-nitroisoxazole 1c, other experimental steps and purification The method is carried out with reference to the steps of Example 1; light yellow oil, 85%ee, 95%yield, 1 H NMR (300MHz, CDCl 3 )δ7.47-7.61 (m, 5H), 4.00-4.07 (m, 3H), 2.01 (s, 1H), 1.46 (d, J = 6.0Hz, 3H), specifically as Figure 4 shown; 13 C NMR (75MHz, CDCl 3 )δ176.6, 157.9, 130.7, 129.7, 129.2, 128.5, 125.7, 64.9, 36.3, 13.9; HRMS (ESI) m / z 249.0877 (M+H + ), calc.forC 12 h 13 N 2 o 4 249.0875.
[0067] The ee value of the compound was analyzed by high performance liquid chromatography (HPLC), CHIRALPAK IE (4.6mm×250mmi.d.), n-hexane / isopropanol=80 / 20 (V / V), flow rate 1.0mL / min, 25°C, 254nm, t R = 7.6 min (small peak) and 9.2 min (large peak).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com