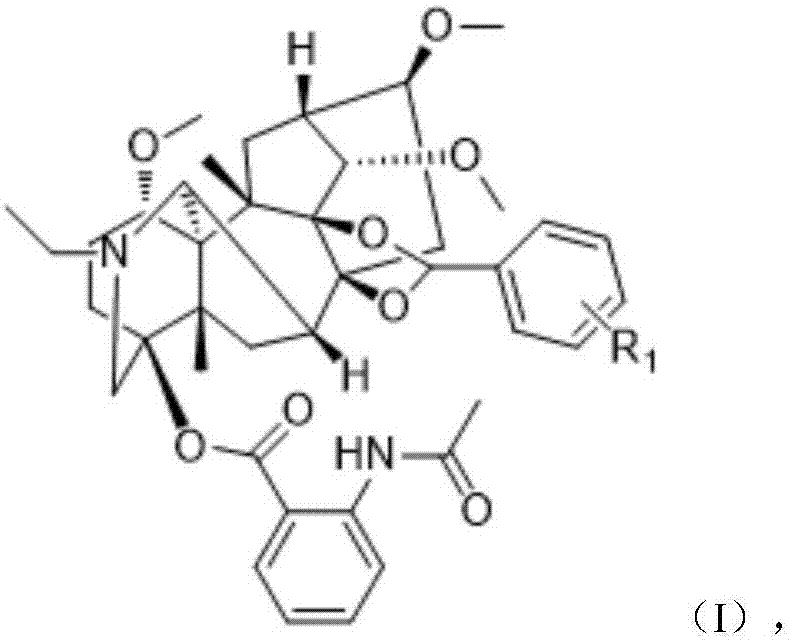
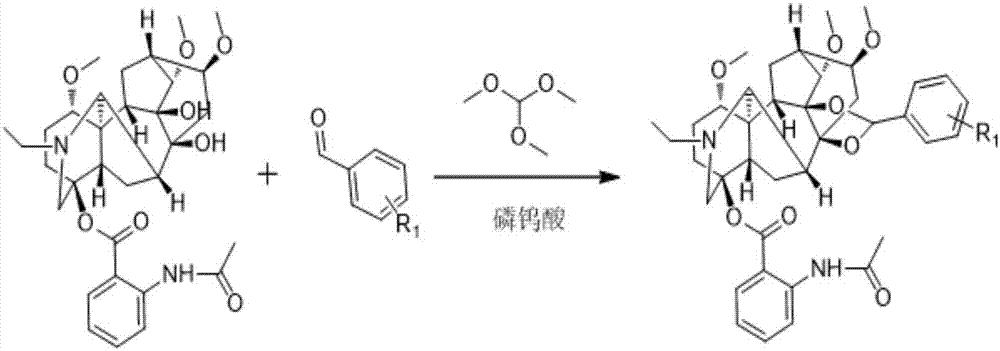
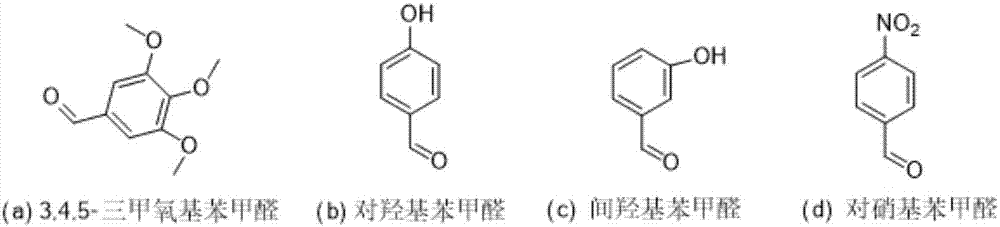
Lappaconitine acetal derivative with antineoplastic activity and synthetic method thereof
A technology with anti-tumor activity and high urine, which is applied in the field of medicinal chemistry, can solve problems such as the influence of drug absorption, side effects of bromide ions, and slow analgesic onset, and achieve low production costs, reduced experimental steps, and high conversion rates. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Condensation products of quinine and 3,4,5-trimethoxybenzaldehyde
[0033] In a 50mL round-bottomed flask, add 100mg of quinine and 67.11mg of 3,4,5-trimethoxybenzaldehyde according to the feeding ratio of 1:2, add a catalytic amount of trimethyl orthoformate and a catalytic amount of phosphotungsten acid, add 25mL toluene as a solvent, stir magnetically at room temperature, and monitor the reaction with thin-layer chromatography. After the reaction is complete, add an appropriate amount of triethylamine to neutralize the reaction solution to neutrality, concentrate the solvent under reduced pressure, and separate and purify by column chromatography to obtain the target compound.
[0034]
[0035] Light yellow amorphous powder 64.91 mg, yield 47.98%. m.p.248~250℃. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.55 (1H, s), 8.27 (1H, d, J = 8.3Hz), 7.85 (1H, d, J = 6.4Hz), 7.57 (1H, t, J = 7.1Hz), 7.27 (2H,s),7.17(1H,t,J=8.1Hz),5.80(1H,s),3.87(12H,s),3.77(3H,s),3.41(1H,d,J...
Embodiment 2
[0036] Condensation product of quinine and p-hydroxybenzaldehyde
[0037] In a 50mL round-bottomed flask, add 100mg of quinine and 41.77mg of p-hydroxybenzaldehyde according to the feeding ratio of 1:2, add a catalytic amount of trimethyl orthoformate and a catalytic amount of phosphotungstic acid, and add 25mL of toluene as a solvent , magnetically stirred at room temperature, and monitored the reaction by thin-layer chromatography. After the reaction was complete, an appropriate amount of triethylamine was added to neutralize the reaction solution to neutrality, the solvent was concentrated under reduced pressure, and separated and purified by column chromatography to obtain the target compound.
[0038]
[0039] White amorphous powder 67.32mg, yield 57.15%. m.p.208~210℃. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.55 (1H, s), 8.27 (1H, d, J = 8.6Hz), 7.85 (1H, d, J = 8.0Hz), 7.76 (2H, d, J = 8.8Hz), 7.57 (1H,t,J=7.9Hz),7.17(1H,t,J=7.7Hz),6.92(2H,d,J=7.8Hz),5.83(1H,s),5.25(...
Embodiment 3
[0041] Condensation product of quinine and m-hydroxybenzaldehyde
[0042] In a 50mL round-bottomed flask, add 100mg of quinolin and 41.77mg of m-hydroxybenzaldehyde according to the feeding ratio of 1:2, add a catalytic amount of trimethyl orthoformate and a catalytic amount of phosphotungstic acid, and add 25mL of toluene as a solvent , magnetically stirred at room temperature, and monitored the reaction by thin-layer chromatography. After the reaction was complete, an appropriate amount of triethylamine was added to neutralize the reaction solution to neutrality, the solvent was concentrated under reduced pressure, and separated and purified by column chromatography to obtain the target compound.
[0043]
[0044] Light yellow amorphous powder 68.92 mg, yield 56.21%. m.p.218~220℃. 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 10.55 (1H, s), 8.27 (1H, d, J = 8.3Hz), 7.85 (1H, d, J = 8.0Hz), 7.58 (1H, dd, J = 6.8, 6.8Hz) ,7.42(1H,t,J=7.7Hz),7.36(1H,d,J=7.5Hz),7.25(1H,m),7.17(1H,t,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com