A kind of fluorination catalyst and preparation method thereof
A fluorination catalyst, fluorination technology, applied in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. The problems of low activity and easy carbon deposition of the catalyst can achieve the effects of excellent by-product control effect, high catalytic activity, and increased specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
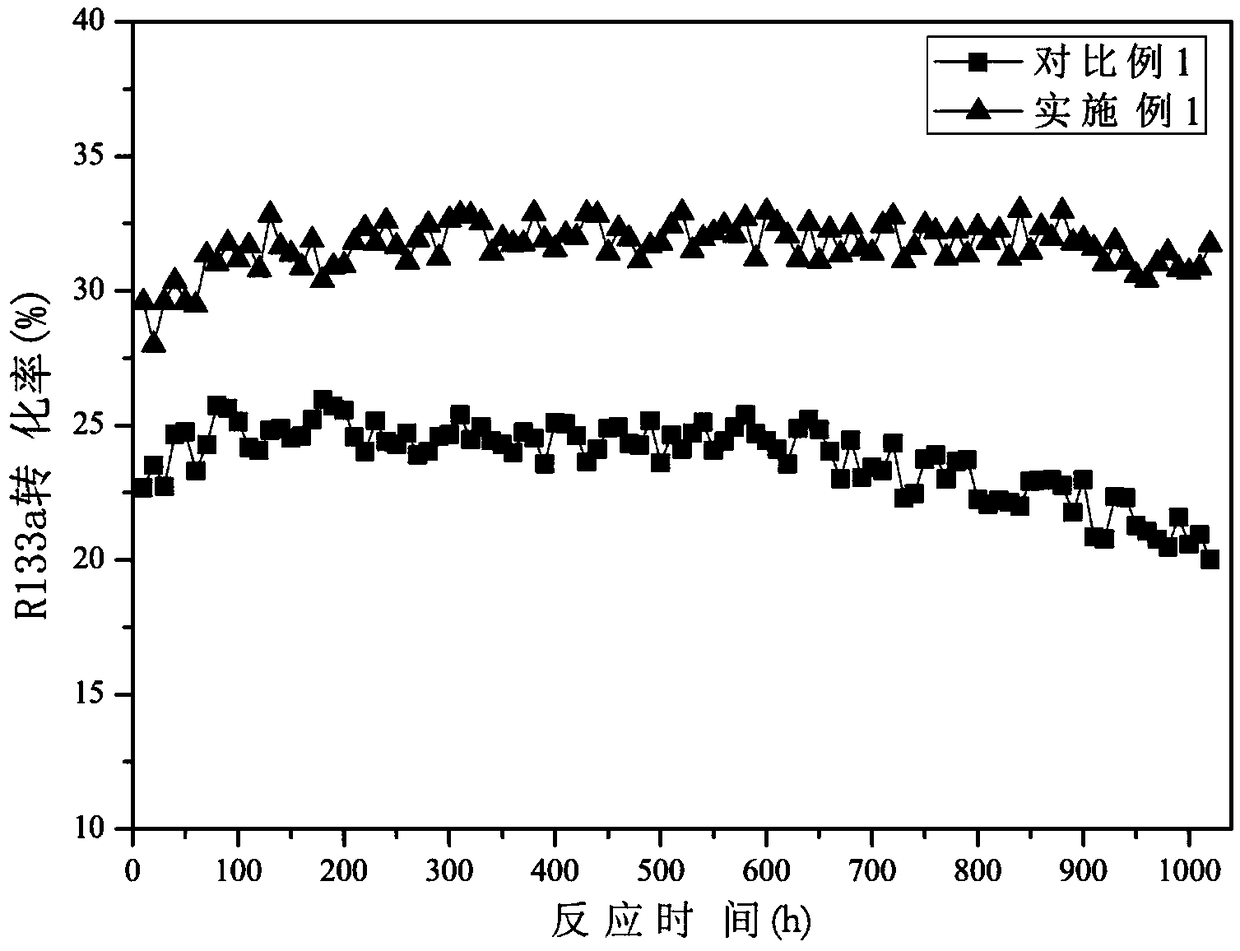
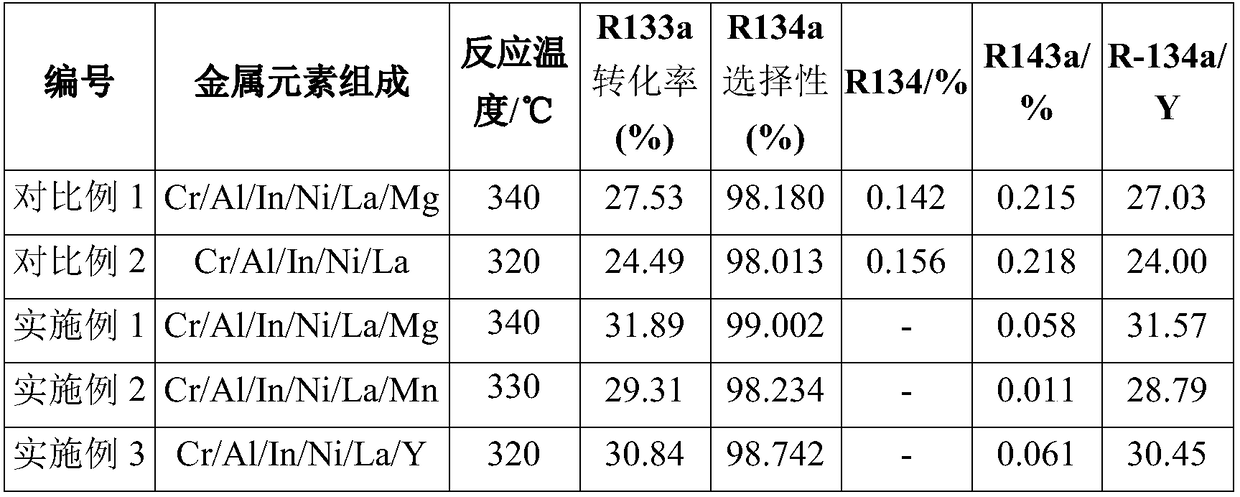
[0049] Weigh 50g CrCl 3 ·6H 2 O, 3.4g AlCl 3 , 0.44g InCl 3 , 1.01g NiCl 2 , 0.61g LaCl 3 Dissolve in 1000mL deionized water, mix the mixed salt solution and ammonia water until the solution pH = 11, filter and wash to obtain the sample, put the sample into an oven for 8 hours at 120°C, add 24g (NH 4 ) 2 CO 3 and transferred to the roaster, at N 2 Calcined at a high temperature of 330° C. for 4 hours in the atmosphere, and the chromium-based precursor was obtained after the roasting.
[0050] Weigh 1.37g MgCl 2 Dissolve in 100 mL deionized water, add the solution dropwise to the vacuum-treated chromium-based precursor, then evaporate the liquid in a water bath, then dry it in an oven at 110 °C for 8 h, and finally place it in a tube furnace under N 2 Calcined at 320° C. for 5 h under atmosphere, crushed and sieved the calcined sample, added graphite and mixed evenly, and pressed into tablets to obtain the catalyst precursor.
[0051] Then, the catalyst precursor was ...
Embodiment 2
[0054] Weigh 50g CrCl 3 ·6H 2 O, 4.53g AlCl 3 , 0.22g InCl 3 , 0.88g NiCl 2 , 0.41g LaCl 3 Dissolve in 1050mL deionized water, mix the mixed salt solution and ammonia water until the solution pH=10, filter and wash to obtain the sample, put the sample into an oven for 100°C and dry it for 10h, then add 32g NH 4 NO 3 and transferred to the roaster, at N 2 Calcined at a high temperature of 340° C. for 5 hours in the atmosphere, and the chromium-based precursor was obtained after the roasting.
[0055] Weigh 0.92g MnCl 2 Dissolve in 110 mL deionized water, add the solution dropwise to the vacuum-treated chromium-based precursor, then evaporate the liquid in a water bath, then dry it in an oven at 120 °C for 9 h, and finally place it in a tube furnace under N 2 Calcined at 350° C. for 4 h under the atmosphere, crushed and sieved the calcined sample, added graphite and mixed evenly, and pressed into tablets to obtain the catalyst precursor.
[0056] Then, the catalyst prec...
Embodiment 3
[0059] Weigh 50g CrCl 3 ·6H 2 O, 5.1g AlCl 3 , 0.55g InCl 3 , 0.76g NiCl 2 , 0.51g LaCl 3 Dissolve in 1100mL deionized water, mix the mixed salt solution and ammonia water until the pH of the solution is 12, filter and wash to obtain the sample, put the sample into an oven and dry it at 110°C for 7 hours, add 18.4g of ammonium tartrate and transfer it to a roasting furnace, in N 2 Calcined at a high temperature of 350° C. for 4 h in the atmosphere, and the chromium-based precursor was obtained after the roasting.
[0060] Weigh 1.01g YCl 4 Dissolve in 100 mL deionized water, add the solution dropwise to the vacuum-treated chromium-based precursor, then evaporate the liquid in a water bath, then dry it in an oven at 110 °C for 10 h, and finally place it in a tube furnace under N 2 Calcined at 330° C. for 3 h under the atmosphere, crushed and sieved the calcined samples, added graphite and mixed evenly, and pressed into tablets to obtain the catalyst precursor.
[0061] T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com