Preparation method for self-cleaning anti-corrosive coating on magnesium alloy surface
A technology for corrosion coating and magnesium alloy, which is applied in the field of preparation of self-healing anti-corrosion coating on the surface of magnesium alloy, and achieves the effects of reduced dosage, good adaptability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
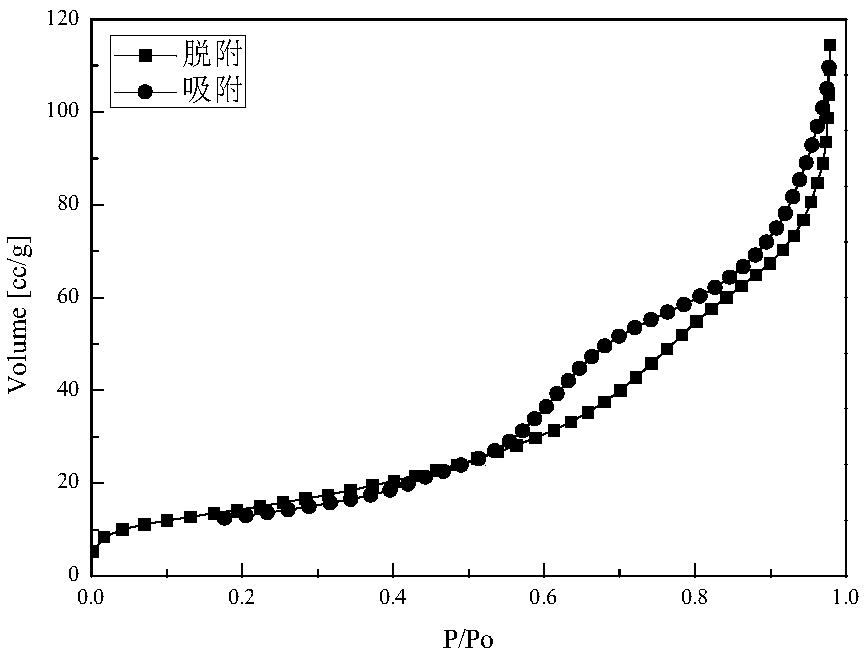
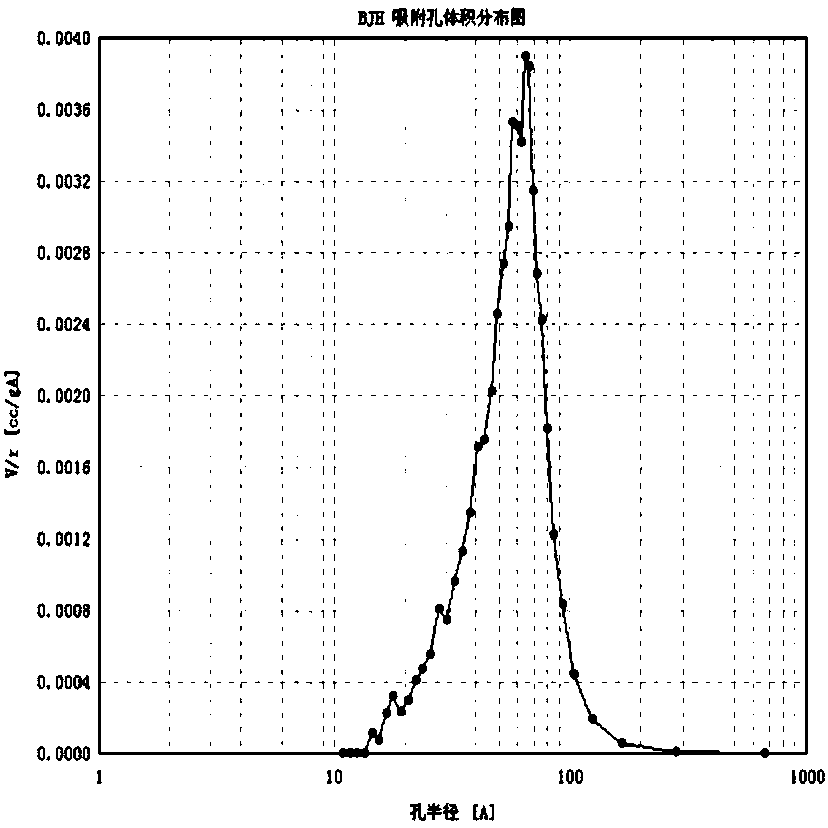
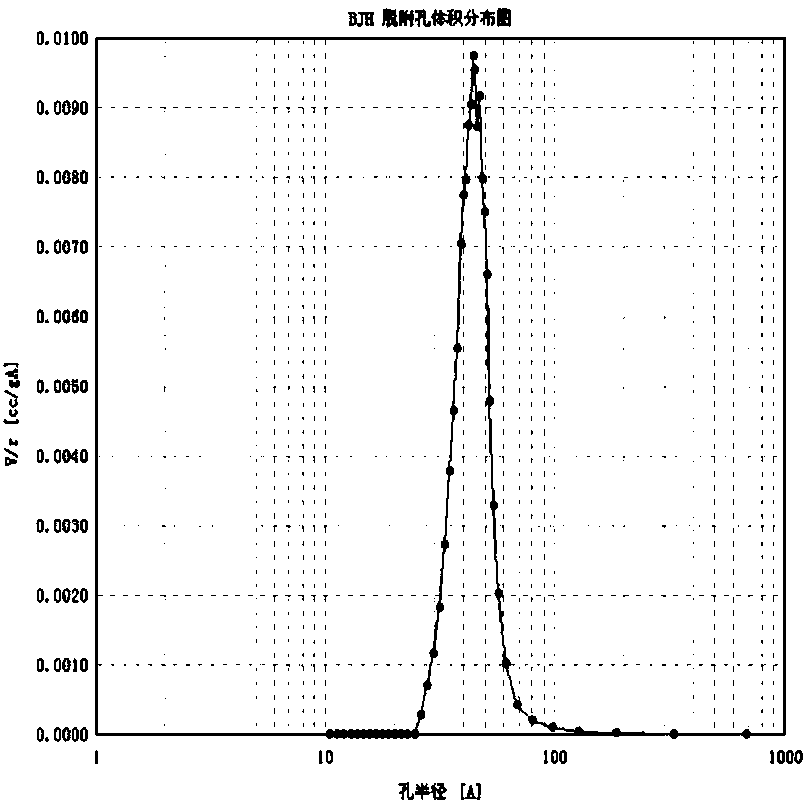
Image
Examples
Embodiment 1
[0039] The first step, the preparation of mesoporous zirconia support: 11g ZrOCl 2 ·8H 2 Dissolve O solid in 40mL distilled water with a concentration of 1.0mol / L; preparation of CTAB solution: dissolve 0.13g CTAB in 40mL distilled water with a concentration of 0.01mol / L; prepare an ammonia solution with a concentration of 1.0mol / L ; Then mix the zirconium oxychloride and CTAB solution and stir, the temperature is not higher than 50°C; add the prepared ammonia solution dropwise to the mixed solution of zirconium oxychloride and CTAB through the dropping funnel; adjust the pH=9.5, drop After heating the ammonia solution to above 70°C, white flocculent precipitates will appear in the solution. After complete precipitation, separate the precipitates with a centrifuge; place the obtained precipitates in a beaker and dry them at 110°C for 10 hours. After being powdered, it was calcined in a muffle furnace at 500°C for 4 hours to prepare a sample for use.
[0040] The second step,...
Embodiment 2
[0044] The first step, the preparation of mesoporous zirconia support: 11g ZrOCl 2 ·8H 2 Dissolve O solid in 40mL distilled water with a concentration of 1.0mol / L; preparation of CTAB solution: dissolve 0.13g CTAB in 40mL distilled water with a concentration of 0.01mol / L; prepare an ammonia solution with a concentration of 1.0mol / L ; Then mix the zirconium oxychloride and CTAB solution and stir, the temperature is not higher than 50°C; add the prepared ammonia solution dropwise to the mixed solution of zirconium oxychloride and CTAB through the dropping funnel; adjust the pH=9.5, drop After heating the ammonia solution to above 70°C, white flocculent precipitates will appear in the solution. After complete precipitation, separate the precipitates with a centrifuge; place the obtained precipitates in a beaker and dry them at 110°C for 10 hours. After being powdered, it was calcined in a muffle furnace at 500°C for 4 hours to prepare a sample for use.
[0045] The second step,...
Embodiment 3
[0049] The first step, the preparation of mesoporous zirconia support: 11g ZrOCl 2 ·8H 2 Dissolve O solid in 40mL distilled water with a concentration of 1.0mol / L; preparation of CTAB solution: dissolve 0.13g CTAB in 40mL distilled water with a concentration of 0.01mol / L; prepare an ammonia solution with a concentration of 1.0mol / L ; Then mix the zirconium oxychloride and CTAB solution and stir, the temperature is not higher than 50°C; add the prepared ammonia solution dropwise to the mixed solution of zirconium oxychloride and CTAB through the dropping funnel; adjust the pH=9.5, drop After heating the ammonia solution to above 70°C, white flocculent precipitates will appear in the solution. After complete precipitation, separate the precipitates with a centrifuge; place the obtained precipitates in a beaker and dry them at 110°C for 10 hours. After being powdered, it was calcined in a muffle furnace at 500°C for 4 hours to prepare a sample for use.
[0050] The second step,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com