Multibranched polyaryletherketone anion-exchange membrane and preparation method thereof
An anion-exchange membrane and polyaryletherketone technology, which is applied in the field of multi-branched polyaryletherketone anion-exchange membrane and its preparation, can solve the problems of inconvenient functional reaction and poor solubility, and achieve a wide range of solvent choices and improve performance , Response operation is simple and fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
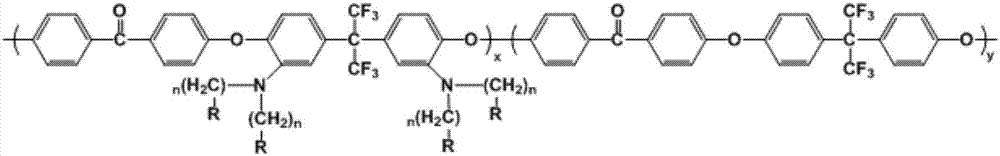
[0037] Synthesis of amino-substituted polyaryletherketone: under nitrogen protection, 4.4081g (20mmol) of difluorobenzophenone, 1.8686g (5mmol) of 2,2-bis(3-amino-4-hydroxyphenyl)hexa Fluoropropane, 1.8813g (15mmol) of hexafluorobisphenol A and 4.1882g (30mmol) of potassium carbonate were added to a 100mL three-necked flask, then 15mL of toluene and 30mL of N-methylpyrrolidone were added to form a mixed solution, and the temperature was gradually raised to 155 °C, reflux until the water is completely removed, remove the toluene, and then keep the temperature constant for 4h. After the reaction is completed, the product is slowly poured into ice water under mechanical stirring, and a filamentous polymer product is precipitated. The product is washed and soaked with ethanol repeatedly, and then dried to obtain a polymer. Dissolve the polymer with N-methylpyrrolidone into a solution with a certain concentration. After the solution is completely dissolved, pour the solution into e...
Embodiment 2
[0044]Synthesis of amino-substituted polyaryletherketone: under nitrogen protection, 4.4081g (20mmol) of difluorobenzophenone, 2.6161g (7mmol) of 2,2-bis(3-amino-4-hydroxyphenyl)hexa Fluoropropane, 4.4602g (13mmol) of hexafluorobisphenol A and 4.1882g (30mmol) of potassium carbonate were added to a 100mL three-necked flask, then 15mL of toluene and 30mL of N-methylpyrrolidone were added to form a mixed solution, and the temperature was gradually raised to 155 °C, reflux until the water is completely removed, remove the toluene, and then keep the temperature constant for 6h. After the reaction is completed, the product is slowly poured into ice water under mechanical stirring, and a filamentous polymer product is precipitated. The product is washed and soaked with ethanol repeatedly, and then dried to obtain a polymer. Dissolve the polymer with N-methylpyrrolidone into a solution with a certain concentration. After completely dissolving, pour the solution into ethanol to precip...
Embodiment 3
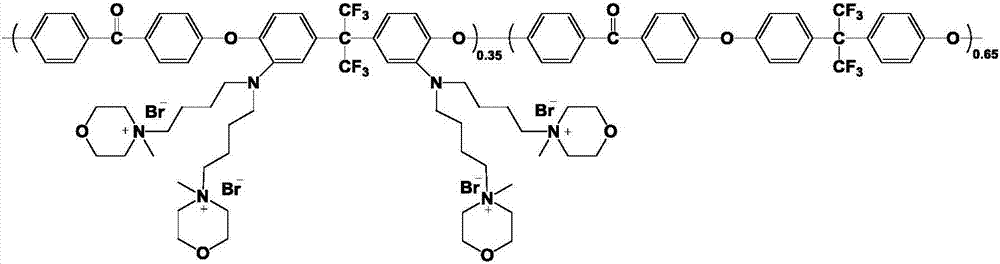
[0051] Synthesis of amino-substituted polyaryletherketone: same as Example 2
[0052] Synthesis of 1-bromohexyl-1-methylpyrrolidine bromide salt ionic liquid: Dissolve 5mL of 1,6-dibromohexane in 15mL of ethyl acetate in a 50mL one-necked flask, add 1.754g of N-methylpyrrolidine, After reacting at 60°C for 24h, a precipitated product in the form of milky white powder was obtained. The product was repeatedly washed with ethyl acetate for 3 to 5 times, and vacuum-dried at 60° C. for 12 hours to obtain a milky white powdery ionic liquid.
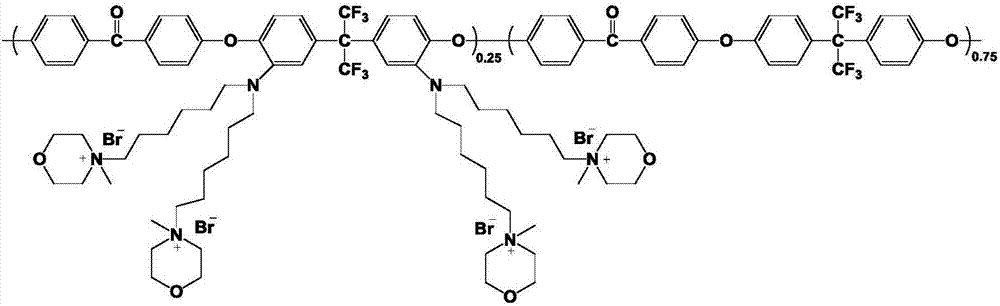
[0053] Preparation of multi-branched polyaryletherketone anion-exchange membrane: Weigh 0.8g of amino-substituted polyaryletherketone polymer into a 100mL three-neck flask under nitrogen protection, dissolve with 20mL DMSO, and add 0.1024g after the polymer is fully dissolved NaH, the reaction system was reacted at 60°C for 7h. Dissolve 1.054g of 1-bromohexyl-1-methylpyrrolidinium bromide salt in 12mL of DMSO, and add it into the flask after ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com