A kind of recombinant human interferon α1b vaginal expansion suppository and preparation method thereof
A technology of recombinant human interferon and vaginal expansive suppository, which is applied in the direction of drug combination, suppository delivery, pharmaceutical formulation, etc., can solve the problems of inability to exert the therapeutic effect of suppositories, waste, and reduced contact area of active ingredients, so as to improve the therapeutic effect, The effect of reducing dosage and reducing frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
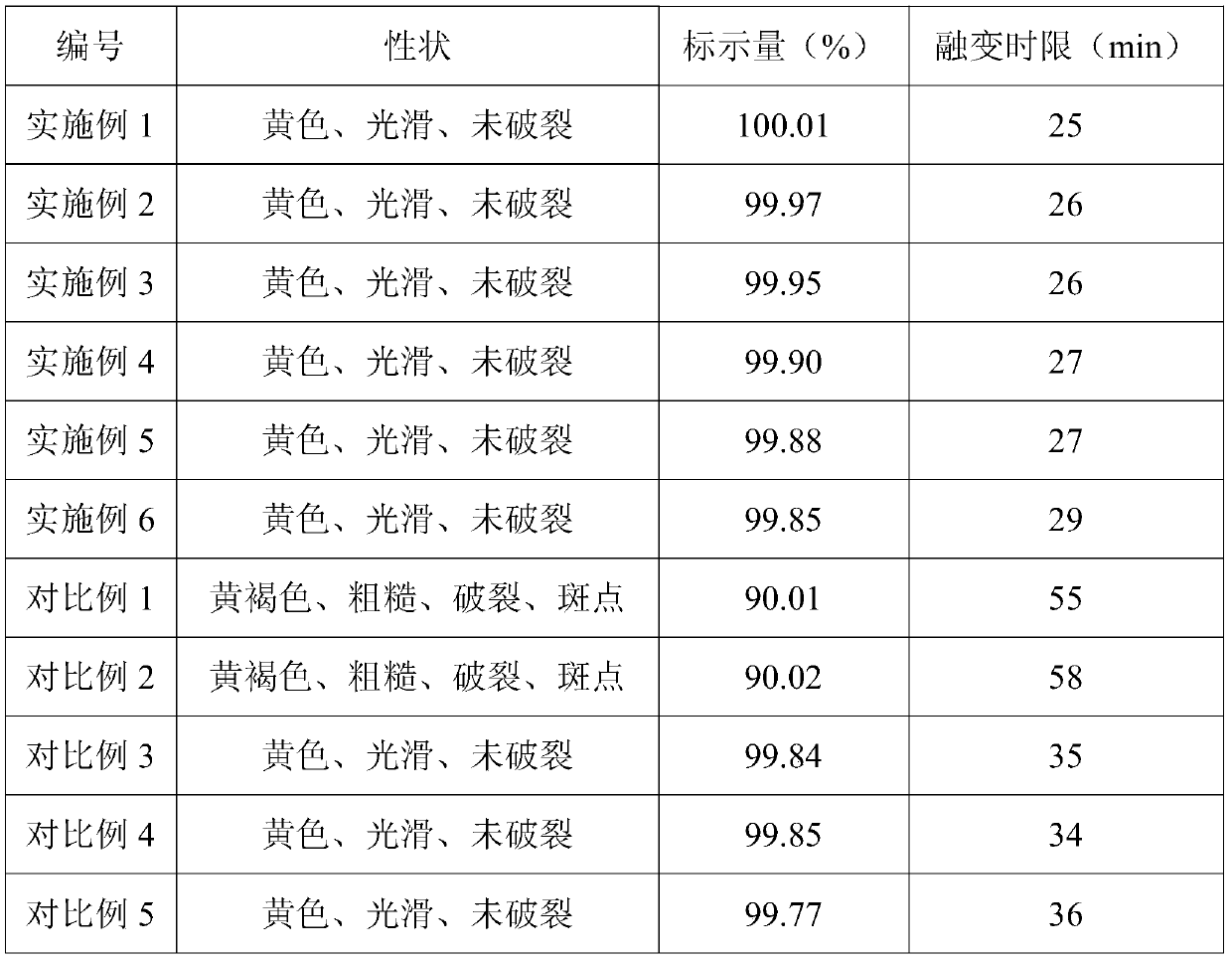
Examples
Embodiment 1
[0042] In this example, the vaginal expansion suppository includes, in parts by weight: 0.1 part of recombinant human interferon α1b, 15 parts of comfrey extract, 8 parts of Viola extract, 5 parts of amethyst extract, 5 parts, 35 parts of polylactic-co-glycolic acid copolymer, 120 parts of a mixture of synthetic fatty acid esters and colloidal compounds, and 300 parts of tampons.
[0043] The preparation method is as follows:
[0044] (1) After mixing the synthetic fatty acid ester and the colloidal compound at a weight ratio of 5:1, heat and melt in a water bath at 65°C to obtain a melt;
[0045] (2) Pour the molten matter described in step (1) into a plug mold, insert a sliver, cool and set the shape, and obtain a matrix plug core;
[0046] (3) After fully mixing the recombinant human interferon α1b and the traditional Chinese medicine composition, spray it on the surface of the matrix plug core described in step (2) in a spray form, and make a suppository after cooling dow...
Embodiment 2
[0049] In this embodiment, the vaginal expansion suppository includes, in parts by weight: 0.05 parts of recombinant human interferon α1b, 18 parts of comfrey extract, 5 parts of Viola extract, 8 parts of amethyst extract, 2 parts, polylactic acid 50 parts, mixture of synthetic fatty acid ester and natural fatty acid ester 80 parts and non-woven fabric 400 parts.
[0050] The preparation method is as follows:
[0051] (1) After mixing the synthetic fatty acid ester and the natural fatty acid ester at a weight ratio of 6:1, heat and melt in a water bath at 64°C to obtain a melt;
[0052] (2) Pour the molten matter described in step (1) into the plug mold, insert the non-woven fabric, cool and set the shape, and obtain the matrix plug core;
[0053] (3) After fully mixing the recombinant human interferon α1b and the traditional Chinese medicine composition, spray it on the surface of the matrix plug core described in step (2) in the form of a spray, and make a suppository after...
Embodiment 3
[0056] In this example, the vaginal expansion suppository includes, in parts by weight: 0.5 parts of recombinant human interferon α1b, 10 parts of comfrey extract, 9 parts of viola extract, 2 parts of amethyst extract, 8 parts, polycaprolactone 30 parts, synthetic fatty acid ester and lipid matrix mixture 150 parts and elastane 150 parts.
[0057] The preparation method is as follows:
[0058] (1) After mixing the synthetic fatty acid ester and the lipid base at a weight ratio of 2:1, place it in a water bath at 68° C. for heating and melting to obtain a melt;
[0059] (2) Pour the molten matter described in step (1) into a plug mold, insert elastic fibers, cool and set the shape, and obtain a matrix plug core;
[0060] (3) After fully mixing the recombinant human interferon α1b and the traditional Chinese medicine composition, spray it on the surface of the matrix plug core described in step (2) in a spray form, and make a suppository after cooling down to 37°C;
[0061] (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com