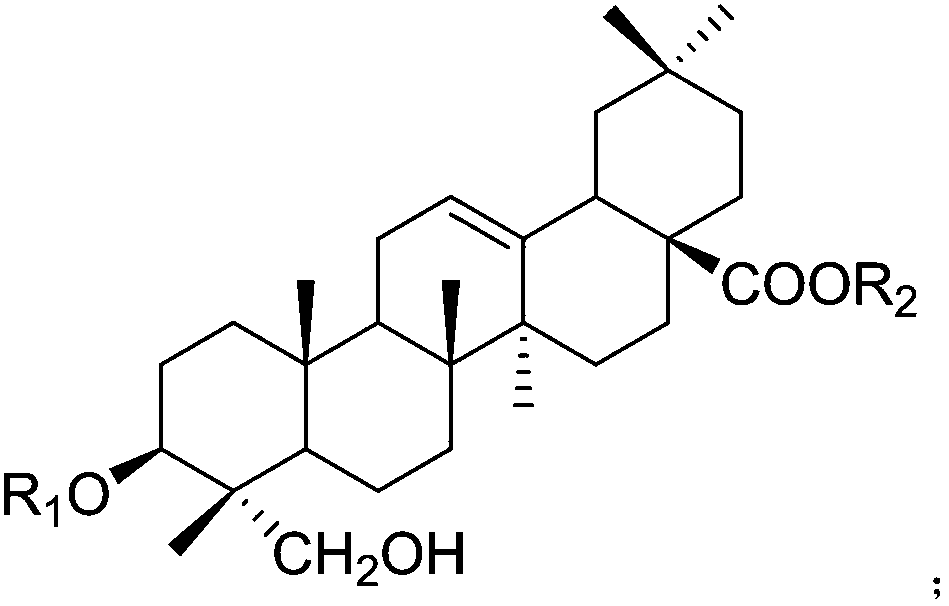
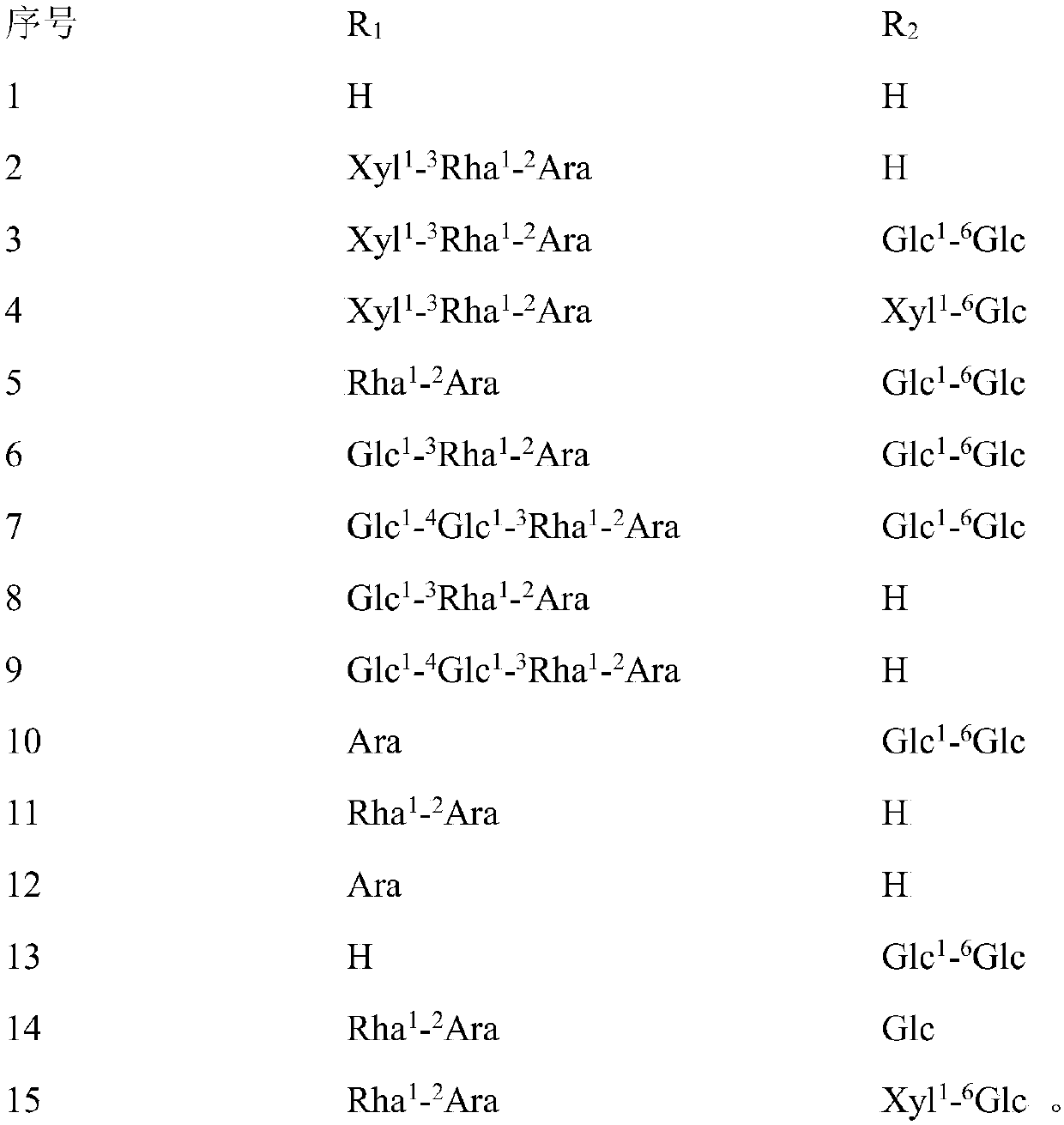
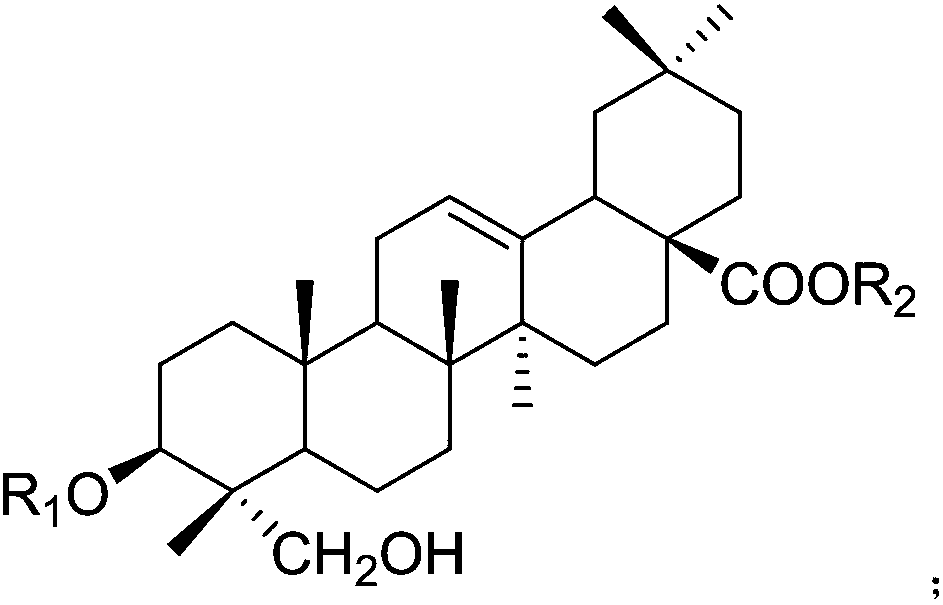
Application of hederagenin and glucoside thereof in preparing anti-virus medicines
A hedera saponin and anti-virus technology, applied in the field of medicine, can solve the problems of hedera saponin and its glycosides that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: The pharmacological effect of hedera saponin and its glycoside
[0042] Anti-respiratory syncytial virus (RSV), herpes simplex virus type 1 (HSV-I) and coxsackie virus type B3 (CVB3) effect (references: Experimental study on the antiviral effect of Shufeng Jiedu Capsules in vivo, New Drugs and Clinical Pharmacology of Traditional Chinese Medicine, Volume 25, Issue 1, January 2014).
[0043]SPF grade ICR mice were randomly divided into 17 groups according to body weight and sex: normal control group, virus control group and hedera saponin or saponin administration group with sequence number 1-15. Except for the normal control group, each mouse in each group was infected with 0.5 mL of RSV at 10LD50, 0.3 mL of HSV-1 at 10LD50, and 0.5 mL of CVB3 at 10LD50 under light anesthesia. Drug intervention was performed 2 hours after nasal drops. The mice in the treatment group were given 30 mg / kg / d helexin or saponin by intragastric administration, and the normal c...
Embodiment 2
[0047] Embodiment 2: Preparation and pharmacological action of an extract containing helexin or its glycosides
[0048] Part 1: Preparation of Extract
[0049] Preparation method one 5kg of dry Lonicera chinense medicinal material, reflux extraction with 70% ethanol for 3 times, 2h each time, filter, combine the filtrate, reclaim the solvent under reduced pressure to obtain extractum (get partly dried to obtain total extract 1), add appropriate amount of water to suspend , filter, and load the sample on D101 macroporous resin, start gradient elution from 10%, 30%, 50%, 70%, 90% ethanol, and monitor whether the eluate contains Decaisoside E, Honeysuckle saponin B or Dipsacus saponin B, any of the three appear in the eluent and start to collect until all three are eluted from the resin and stop collecting. Concentrate and dry this part of the eluent to obtain the extract of Lonicera tomentosa Object 1.
[0050] Preparation method two 5kg of dry Lonicera chinensis medicinal ...
Embodiment 3
[0063] Embodiment 3: pharmaceutical preparation
[0064] 1. Tablet: 5g of helexin or saponin or Lonicera tomentosa extract of serial number 1-15, 50g of starch, 3g of magnesium stearate. Preparation process: take the hedera saponin or saponin or the extract of Lonicera tomentosa with the serial number 1-15, add starch and magnesium stearate, mix evenly, make granules, dry, and compress into tablets.
[0065] 2. Capsules: 5g of hedera saponin or saponin or Lonicera tomentosa extract with serial number 1-15, 50g of starch, 3g of magnesium stearate. Preparation process: take the hedera saponin or saponin or Lonicera tomentosa extract of serial number 1-15, add starch and magnesium stearate, mix evenly, make granules, dry, and pack into capsules.
[0066] 3. Injection: 1g of hedera saponin or saponin or Lonicera tomentosa extract with serial number 1-15, appropriate amount of sodium chloride for injection. Preparation process: take the hedera saponin or saponin or Lonicera chrys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com