Phenazine-1-carboxylic acid bisamide compounds and application thereof
A technology of carboxylic acid bisamides and compounds, which is applied in the field of pesticide compound preparation, can solve the problems that it is difficult to develop into diversified dosage forms, and achieve a broad-spectrum bactericidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The synthetic method of phenazine-1-carboxylic acid bisamide compound of the present invention comprises the following steps:
[0031]
[0032] Below in conjunction with specific example, further set forth the present invention. It should be understood that these examples are only for illustrating the present invention and are not intended to limit the scope of the present invention. The experimental methods that do not indicate the specific implementation conditions in the following examples are usually in accordance with conventional conditions, or in accordance with the conditions suggested by the manufacturer. Percentages and parts are by mass unless otherwise indicated.
[0033] The synthesis method of the phenazine-1-carboxylic acid bisamide compound of the present invention is described below with the synthesis conditions and methods of some example compounds.
example 1
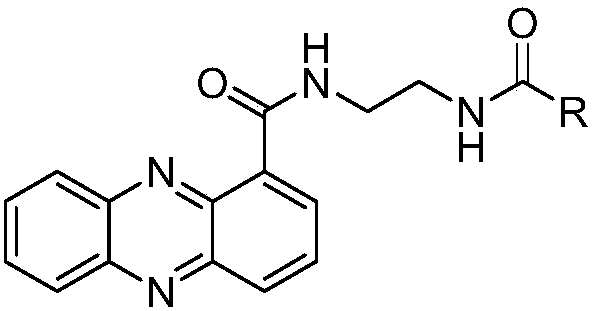
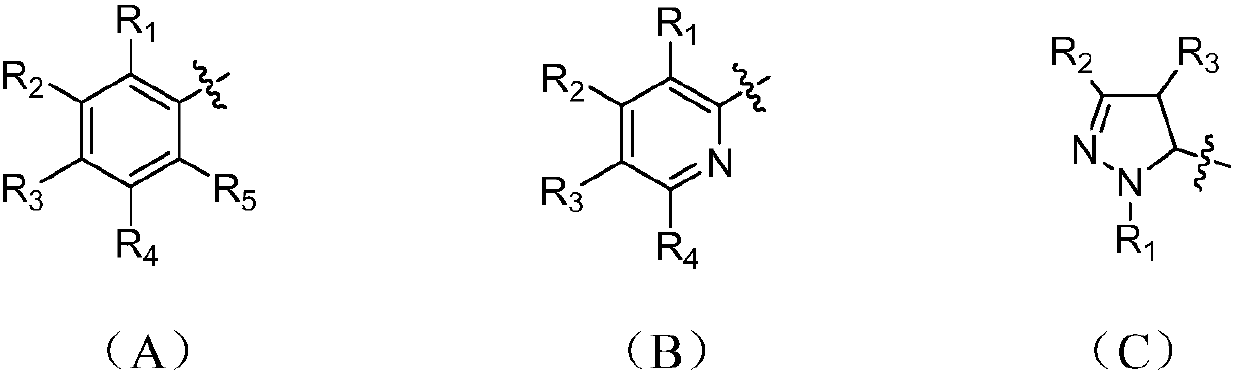
[0034] Example 1: N-(2-phenacylethylamino)phenazine-1-carboxamide (R is a substituted phenyl group (A) in the general formula (I), R 1 , R 2 , R 3 , R 4 and R 5 For the synthesis of H):
[0035] 1) Synthesis of phenazine-1-formyl chloride:
[0036]
[0037] Add 2.5 g (11.2 mmol) of phenazine-1-carboxylic acid, 30 ml of dichloromethane, 1-2 drops of DMF into a 100 ml single-port reaction flask, slowly add 3.0 g of oxalyl chloride (to prevent flushing), and then heat to reflux React until the solid of Shenzimycin completely disappears, continue the reflux reaction for 2-3 hours, remove the solvent on a rotary evaporator, add a small amount of dichloromethane to dissolve, and then spin dry to remove excess oxalyl chloride as much as possible. Then add a certain amount of dichloromethane to dissolve and use in the next step.
[0038] 2) Synthesis of phenazine-1-carboxylic acid methyl ester:
[0039]
[0040] Add 50mL of anhydrous methanol into a 100mL three-necked fla...
example 2
[0051] Example 2: Synthesis of N-(2-butyrylethylamino) phenazine-1-carboxamide (R is a saturated C3 substituted linear alkane group in general formula (I):
[0052] 1) Synthesis of butyryl chloride:
[0053]
[0054] Add 0.88g (10.0mmol) of benzoic acid into a 100ml single-port reaction flask, slowly add 15ml of thionyl chloride dropwise under stirring, then heat and reflux until the solid disappears completely, continue the reflux reaction for 2-3 hours, and place on a rotary evaporator Remove the solvent, add a small amount of toluene to dissolve, and then spin dry to remove excess thionyl chloride as much as possible. Then add a certain amount of dichloromethane to dissolve and use in the next step.
[0055] 2) Synthesis of N-(2-butyrylethylamino)phenazine-1-carboxamide:
[0056]
[0057] In a 100mL three-necked flask, add 2.66g (10.0mol) of N-(2-phenacylethylamino)phenazine-1-carboxamide and 50mL of dichloromethane, stir to completely dissolve the solid, and cool t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com