Amniotic-membrane lacrimal passage repair support and manufacturing method thereof
A manufacturing method and technology for membrane lacrimal ducts, applied in the field of biomedicine, can solve the problems of lacrimal duct tissue damage, lack of repair, secondary damage, etc., and achieve the effects of low recurrence rate, convenient transportation, and prevention of scar formation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
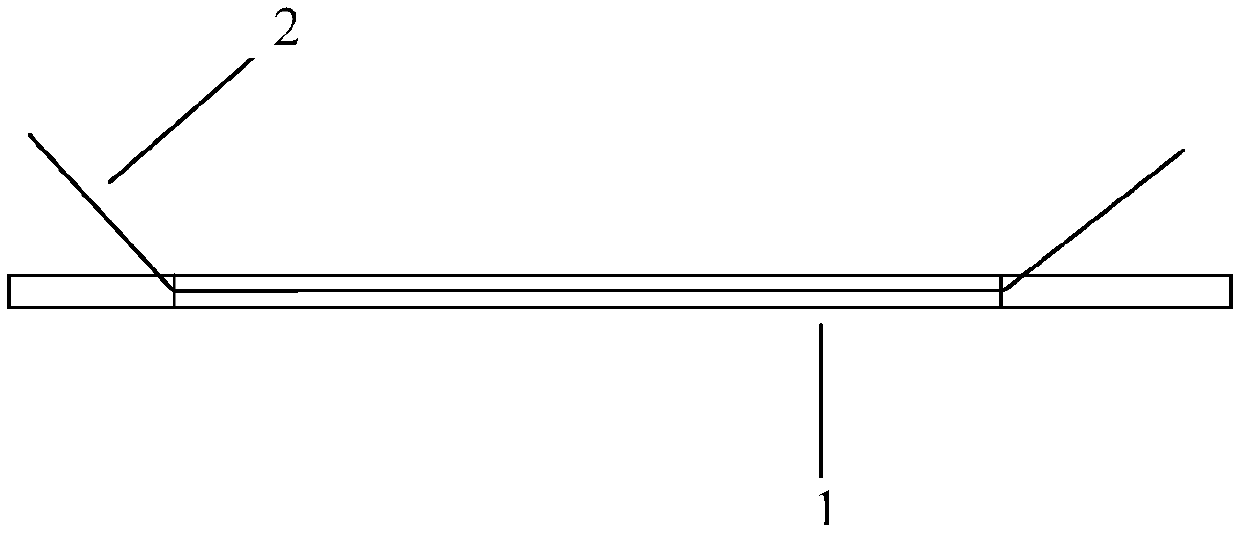
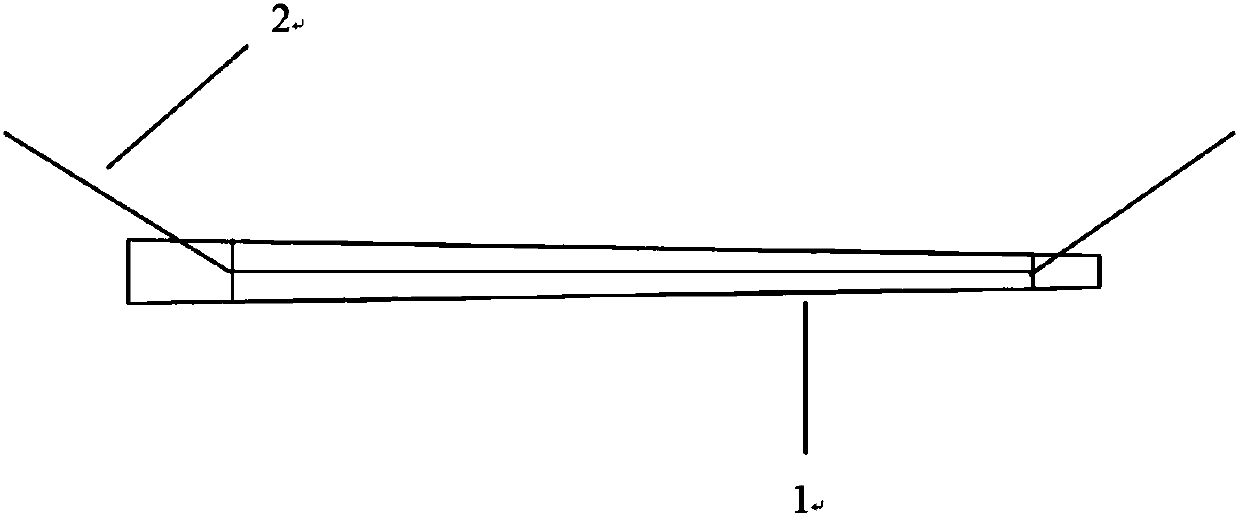
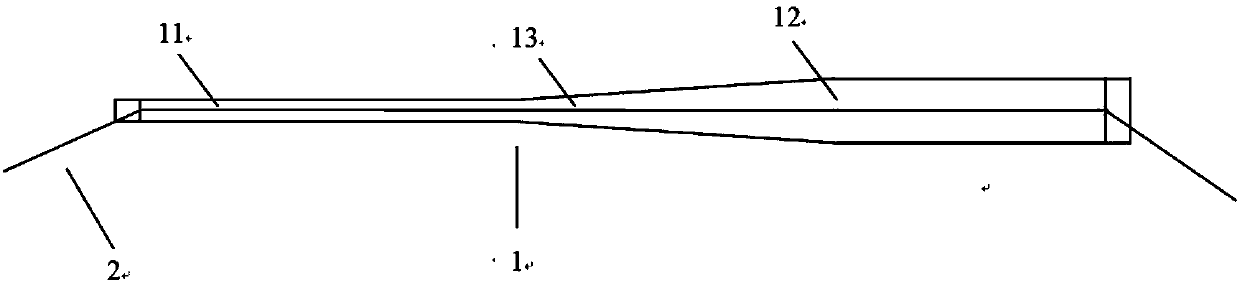
[0033] like figure 1 As shown, the present invention provides a stent for repairing the amniotic membrane lacrimal duct, comprising an amniotic stent body 1 made of amnion treated by freeze-drying, and a medical suture 2 respectively connected to two ends of the amniotic stent body 1. The distance between the suture nodes and the top of the amniotic membrane is 3mm±1mm, the length of the amniotic membrane support body 1 between the two suture nodes is slightly longer than the length of the medical suture 2 between the two suture nodes, and the amniotic membrane support during traction The main body 1 is not stressed, so as to play a protective role and avoid breaking or breaking the amniotic membrane stent main body 1; The body 1 has a constant diameter along its length.
[0034] The manufacturing method of the above-mentioned amniotic lacrimal duct repair stent is as follows, including the following steps:
[0035] 1) Amniotic membrane sampling and cleaning: Amniotic membr...
Embodiment 2
[0042] like figure 1 As shown, the present invention provides a stent for repairing the amniotic membrane lacrimal duct, comprising an amniotic stent body 1 made of amnion treated by freeze-drying, and a medical suture 2 respectively connected to two ends of the amniotic stent body 1. The distance between the suture nodes and the top of the amniotic membrane is 4mm±1mm, the length of the amniotic stent body 1 between the two suture nodes is slightly longer than the length of the medical suture 2 between the two suture nodes, and the amniotic stent is stretched during traction. The main body 1 is not stressed, so as to play a protective role and avoid breaking or breaking the amniotic membrane stent main body 1; The body 1 has a constant diameter along its length.
[0043] The manufacturing method of the above-mentioned amniotic lacrimal duct repair stent is as follows, including the following steps:
[0044] 1) Amniotic membrane sampling and cleaning: Amniotic membrane samp...
Embodiment 3
[0051] like figure 1 As shown, the present invention provides a stent for repairing the amniotic membrane lacrimal duct, comprising an amniotic stent body 1 made of amnion treated by freeze-drying, and a medical suture 2 respectively connected to two ends of the amniotic stent body 1. The distance between the suture nodes and the top of the amniotic membrane is 1 mm, and the length of the amniotic membrane support body 1 between the two suture nodes is slightly greater than the length of the medical suture 2 between the two suture nodes. During the traction process, the amniotic membrane support body 1 No force, so as to play a protective role, avoiding the amniotic membrane stent body 1 from being broken or damaged; Has a constant diameter along its length.
[0052] The manufacturing method of the above-mentioned amniotic lacrimal duct repair stent is as follows, including the following steps:
[0053] 1) Amniotic membrane sampling and cleaning: Amniotic membrane sampling ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com