Solid electrolyte of lithium ion battery and preparation method of solid electrolyte as well as lithium ion battery
A solid-state electrolyte, lithium-ion battery technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as narrow electrochemical window of solid-state electrolytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The present invention also provides a method for preparing the above-mentioned solid electrolyte, the steps of which include:
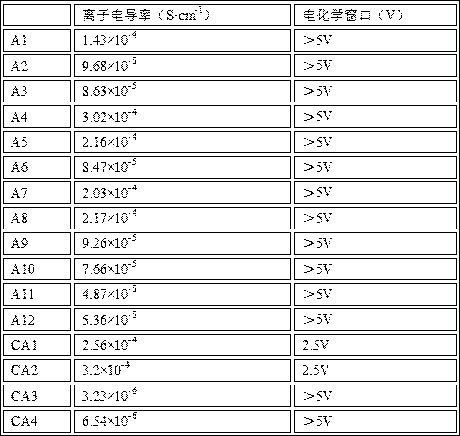
[0023] S1. Obtain the core material, the core material includes Li 1+x m x Ti 2-x (PO 4 ) 3 , wherein, M is selected from at least one of Al, La, Cr, Ga, Y or In, 0.05£x £ 0.4;
[0024] S2. Mix the core material with a solution containing the lithium source of the shell, the phosphate salt of the shell, the silicon source and the boron source, adjust the pH value to 8-11, and dry to obtain the precursor material. The solution used to adjust the pH value is generally ammonia water, so that the solution is gel-like coated on the outer surface of the core material, and then dried to obtain the precursor material. When mixing the solution containing the lithium source of the outer shell, the phosphate salt of the outer shell, the source of silicon and the boron source with the core material, the relative content of the two can be varied withi...
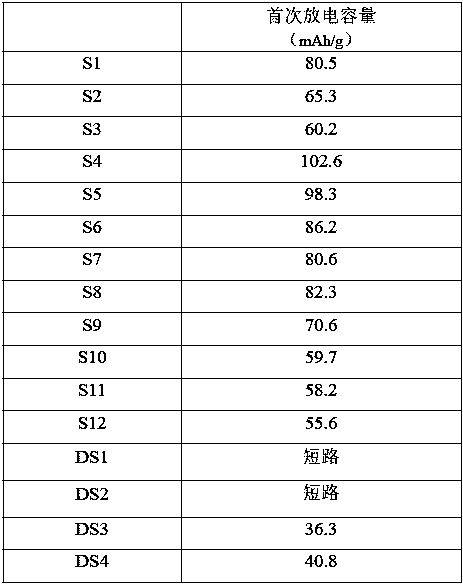
Embodiment 1
[0045] This example is used to illustrate the solid electrolyte disclosed in the present invention and its preparation method.
[0046] 1. Weigh 2.10gLi respectively 2 CO 3 Powder, 0.26g Al 2 o 3 Powder, 7.86g TiO 2 powder and 17.86gNH 4 h 2 PO 4 , ball milled and mixed evenly.
[0047] 2. Put the uniformly mixed powder in step 1 into an alumina crucible, and then place it in a muffle furnace for preliminary calcination at 800°C for 6 hours. After cooling, the chemical formula is Li 1.1 al 0.1 Ti 1.9 (PO 4 ) 3 The core material powder. Its average particle diameter is 5 μm.
[0048] 3. Weigh 0.07g LiOH, 0.18g H 3 BO 3 , 0.34g NH 4 h 2 PO 4 and 0.15g ethyl orthosilicate were dispersed in deionized water to form an aqueous solution, and 20g of the above-prepared Li 1.1 al 0.1 Ti 1.9 (PO 4 ) 3 Inner core material powder, vigorously stir evenly, and adjust the pH value of the system to 11 with 2mol / L ammonia water to form a uniform gel coated on Li 1.1 al ...
Embodiment 2
[0051] This example is used to illustrate the solid electrolyte disclosed in the present invention and its preparation method.
[0052] The solid electrolyte sheet A2 was prepared by the same method steps as in Example 1. The difference lies in weighing 2.07gLi 2 CO 3 Powder, 0.57g Y 2 o 3 Powder, 7.73g TiO 2 powder and 17.58g NH 4 h 2 PO 4 Made Li 1.1 Y 0.1 Ti 1.9 (PO 4 ) 3 The core material powder has an average particle diameter of 5 μm. Add 20g of the above-prepared Li to the aqueous solution 1.1 Y 0.1 Ti 1.9 (PO 4 ) 3 The thickness of the shell material formed by the core material powder is 25nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Electronic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com