Aerosol for treating diseases of respiratory system and preparation method of aerosol
A respiratory disease and aerosol technology, which is applied in the field of aerosols for the treatment of respiratory diseases and its preparation, can solve problems such as undeveloped dosage forms, and achieves the effects of treating respiratory diseases with stable quality and remarkable effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
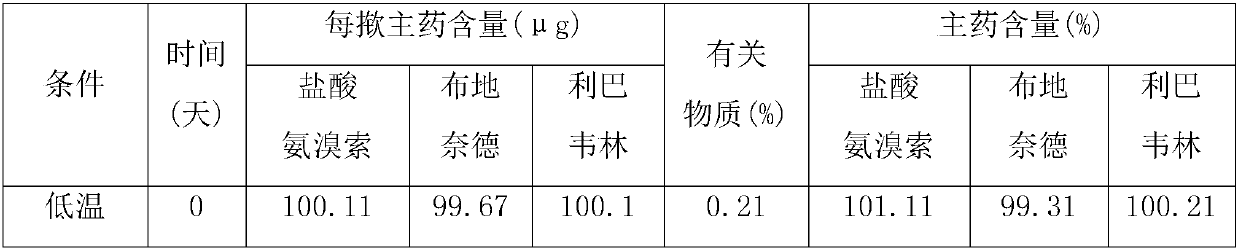
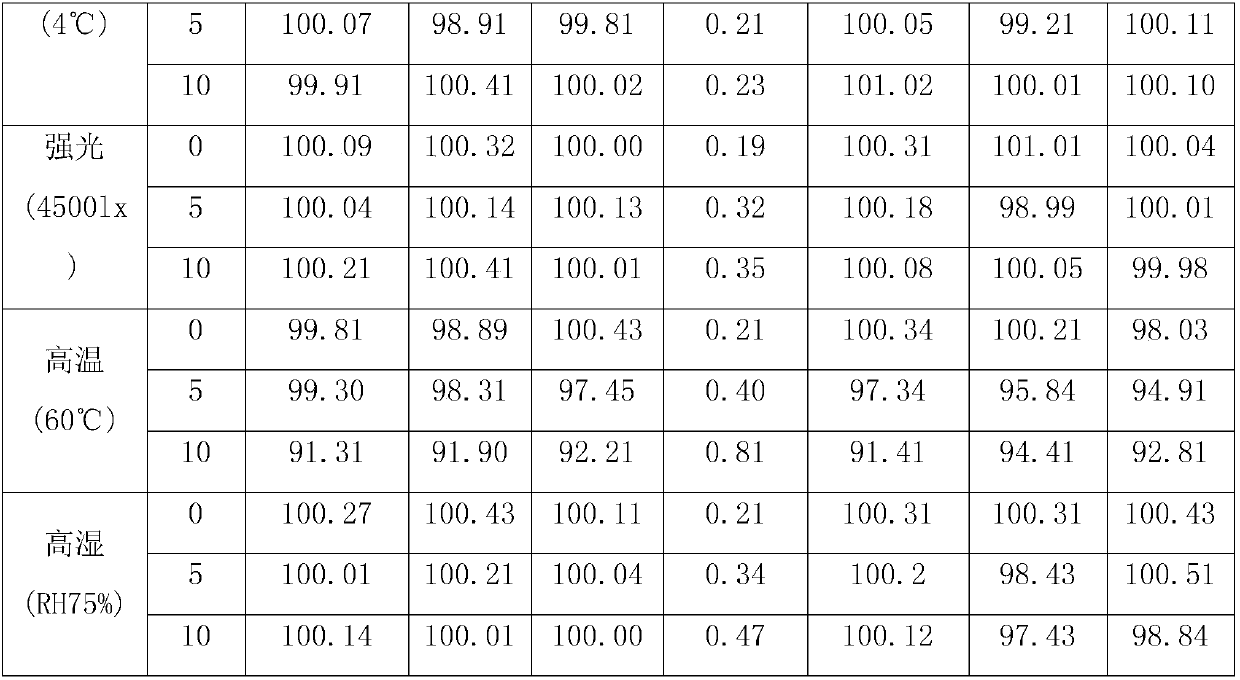
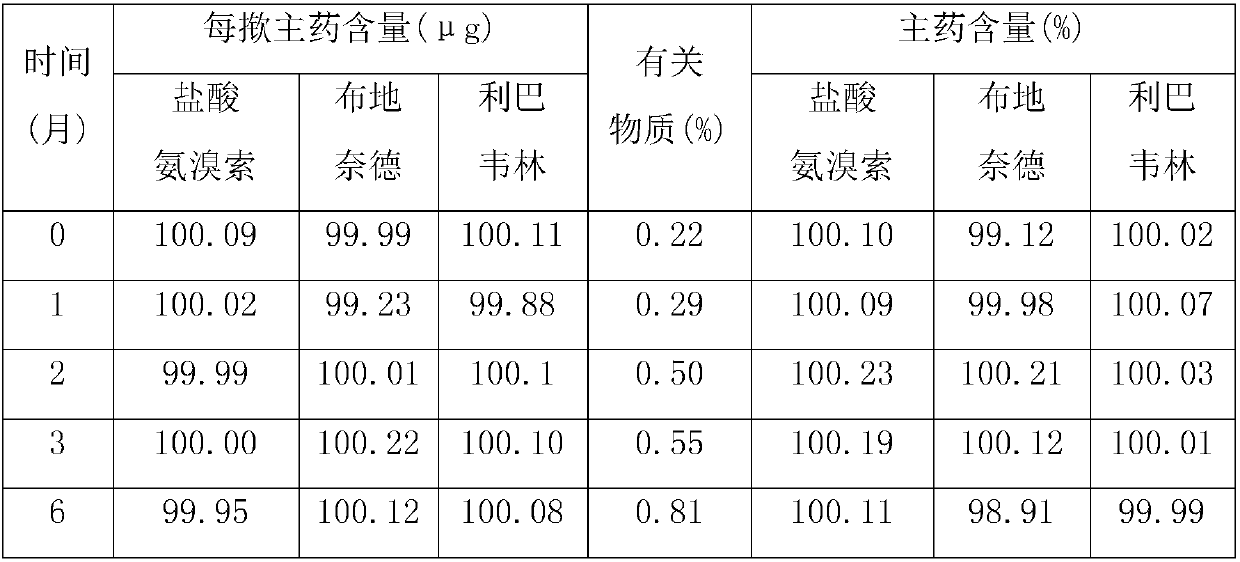
Image
Examples
Embodiment 1
[0017] Weigh each component according to the following weight ratio: Ambroxol hydrochloride 30g, budesonide 20g, ribavirin 20g, glycerin 100g, sorbitan trioleate 15g, PVP 15g, sorbic acid 0.5g, peppermint essence 0.5g , crush ambroxol hydrochloride, budesonide and ribavirin through a 200-mesh sieve, add purified water and stir to dissolve evenly, add glycerin, sorbitan trioleate, PVP, sorbic acid and sodium saccharin, and use a high-pressure homogenizer Stir homogeneously, then add mint essence, continue to stir evenly, fill with valves, fill with propellant dichlorodifluoroethane, check for leaks and weigh, install a booster, and the product is ready.
Embodiment 2
[0019] Each component was weighed according to the following weight ratio: 50g of ambroxol hydrochloride, 40g of budesonide, 30g of ribavirin, 200g of glycerin, 25g of sorbitan trioleate, 25g of PVP, 1g of sorbic acid, 2g of peppermint essence, and Ambroxol hydrochloride, budesonide and ribavirin were crushed through a 200-mesh sieve, added to purified water, stirred and dissolved evenly, added glycerin, sorbitan trioleate, PVP, sorbic acid and sodium saccharin, and homogenized with a high-pressure homogenizer Stir, then add peppermint essence, continue to stir evenly, fill with valves, fill with propellant dichlorodifluoroethane, check for leaks and weigh, install a booster, and you have it.
Embodiment 3
[0021] Weigh each component according to the following weight ratio: 40g of ambroxol hydrochloride, 30g of budesonide, 25g of ribavirin, 150g of glycerin, 20g of sorbitan trioleate, 20g of PVP, 1g of sorbic acid, 1g of peppermint essence, and Ambroxol hydrochloride and albuterol sulfate were crushed through a 200-mesh sieve, added to purified water, stirred and dissolved evenly, and then ethanol, propylene glycol, sodium laurylsulfonate, PVP, vitamin C and methyl p-hydroxybenzoate were added, and high-shear Stir homogeneously for about 30 minutes under the mixer, add apple essence, continue stirring for about 20 minutes, fill with valves, fill with propellant F12, check for leaks and weigh, install a booster, and the product is ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com