Method for synthesizing mephenesin carbamate by using carbon dioxide, amine and aryl diazoacetate
A kind of technology of diazoacetate, carbamate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
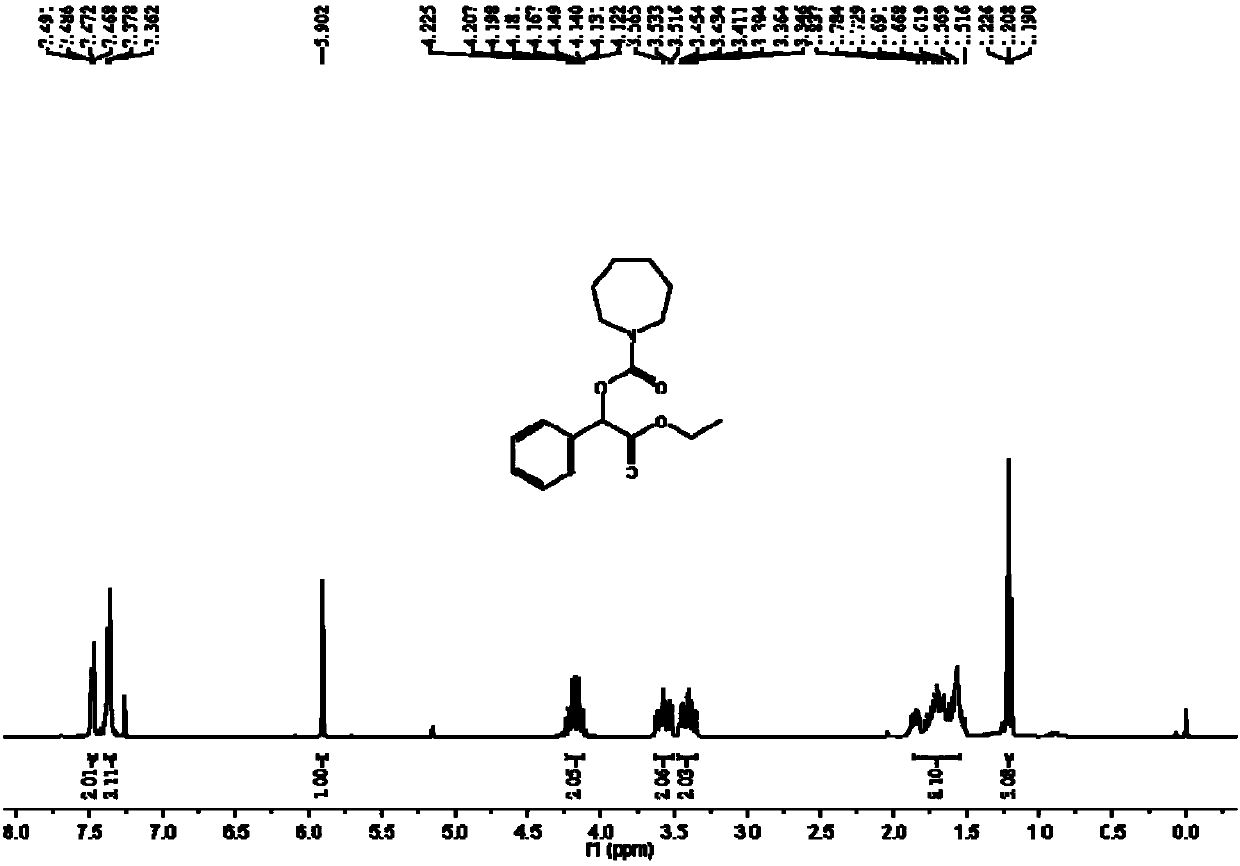
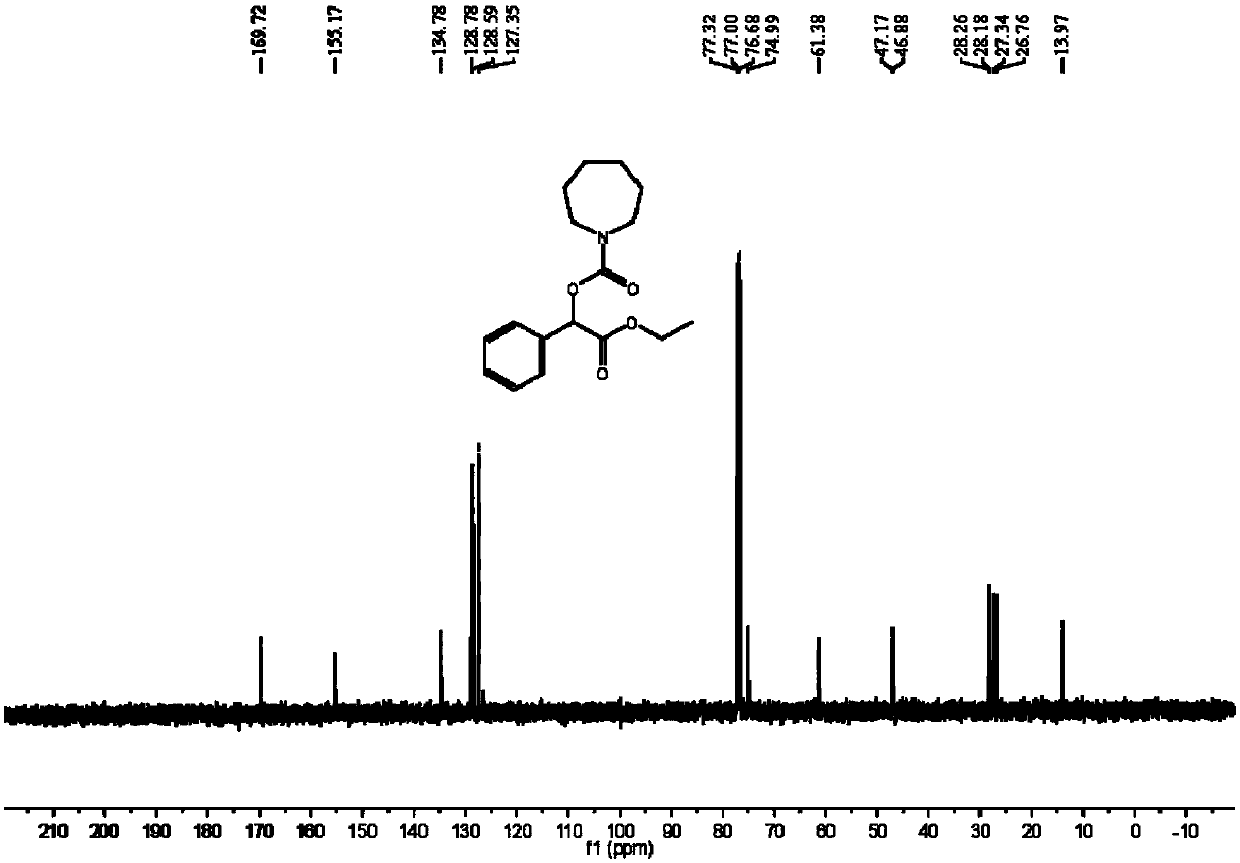
[0047] The synthesis of azepane-1-carboxylic acid (2-ethoxy-2-oxo-1-phenyl) ethyl ester comprises the steps:
[0048] In the autoclave, add 0.2mmol ethyl 2-diazo-2-phenylacetate, 0.02mmol silver carbonate, 3mL acetonitrile and 0.6mmol cycloheximide, and then slowly fill in carbon dioxide to make the pressure reach 4MPa, at 80 ℃ and stirred for 12 hours; after the reaction, stop heating and stirring, cool to room temperature, slowly release unreacted carbon dioxide to normal pressure, dilute the reaction solution with ethyl acetate, filter, then distill off the solvent under reduced pressure to obtain a crude product, and then Separation and purification by column chromatography, the eluent of column chromatography is sherwood oil with a volume ratio of 20:1: ethyl acetate mixed solvent to obtain the target product azepane-1-carboxylic acid (2-ethoxy -2-oxo-1-phenyl)ethyl ester, yield 45%.
[0049] The hydrogen spectrogram and the carbon spectrogram of described obtaining prod...
Embodiment 2
[0054] The synthesis of azepane-1-carboxylic acid (2-ethoxy-2-oxo-1-phenyl) ethyl ester comprises the steps:
[0055] In the autoclave, add 0.2mmol ethyl 2-diazo-2-phenylacetate, 0.02mmol silver fluoride, 3mL acetonitrile and 0.6mmol cycloheximide, and then slowly fill in carbon dioxide to make the pressure reach 4MPa. Stir and react at 80°C for 12 hours; after the reaction, stop heating and stirring, cool to room temperature, slowly release unreacted carbon dioxide to normal pressure, dilute the reaction solution with ethyl acetate, filter, and then distill off the solvent under reduced pressure to obtain a crude product. Separation and purification by column chromatography, the eluent of column chromatography is sherwood oil with a volume ratio of 20:1: ethyl acetate mixed solvent to obtain the target product azepane-1-carboxylic acid (2-ethoxy yl-2-oxo-1-phenyl)ethyl ester, yield 78%.
[0056] The hydrogen spectrogram and the carbon spectrogram of the resulting product are...
Embodiment 3
[0059] The synthesis of azepane-1-carboxylic acid (2-ethoxy-2-oxo-1-phenyl) ethyl ester comprises the steps:
[0060] In the autoclave, add 0.2mmol ethyl 2-diazo-2-phenylacetate, 0.01mmol silver acetate, 3mL acetonitrile and 0.2mmol cycloheximide, then slowly fill in carbon dioxide to make the pressure reach 6MPa, at 80 ℃ and stirred for 12 hours; after the reaction, stop heating and stirring, cool to room temperature, slowly release unreacted carbon dioxide to normal pressure, dilute the reaction solution with ethyl acetate, filter, then distill off the solvent under reduced pressure to obtain a crude product, and then Separation and purification by column chromatography, the eluent of column chromatography is sherwood oil with a volume ratio of 20:1: ethyl acetate mixed solvent to obtain the target product azepane-1-carboxylic acid (2-ethoxy -2-oxo-1-phenyl)ethyl ester, yield 18%.
[0061] The hydrogen spectrogram and the carbon spectrogram of the resulting product are show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com