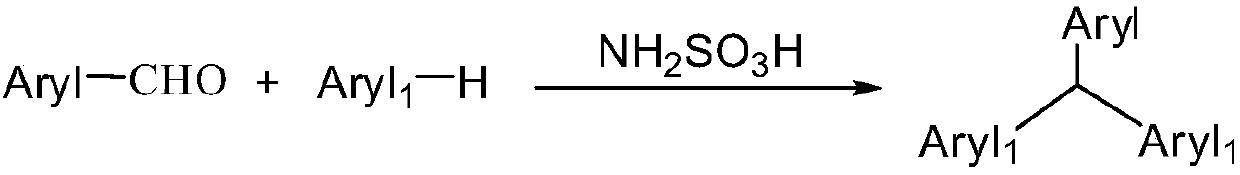
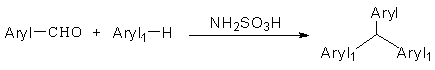Synthesis method of triarylmethane and derivative thereof under solvent-free condition
An arylmethane and a synthesis method technology, applied in the field of organic compound synthesis, can solve problems such as high cost and inability to recover, and achieve the effects of small environmental impact, cheap raw material range and good universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1.5, the synthesis of 5'-(benzyl) bi-(2-methylfuran):
[0017] Add 1mmol (0.103mL) benzaldehyde, 2mL 2-methylfuran, and 0.1mmol sulfamic acid into a round bottom flask, then heat to 70°C with an oil bath under magnetic stirring, and react for 6 hours. Remove the oil bath and cool down to room temperature; the catalyst is filtered out, the mother liquor is concentrated by a rotary evaporator, and the residue is separated by silica gel thin-layer chromatography using petroleum ether as a developing solvent. Pure triarylmethane was obtained with an isolated yield of 90.0%. The nuclear magnetic analysis data of this compound are as follows:
[0018] 1 H NMR (400MHz, CDCl 3 )δ:7.31-7.21(m,5H),5.87-5.85(m,4H),5.33(s,1H),2.23(s,6H). 13 C NMR (100MHz, CDCl 3 )δ: 152.8, 151.4, 140.0, 128.4, 128.3, 126.9, 108.1, 106.0, 45.1, 13.6. MS: m / z 252.10 [M] + ..
Embodiment 2
[0019] Embodiment 2.5, the synthesis of 5'-(p-methylbenzyl) bi-(2-methylfuran):
[0020] Add 1mmol (0.103mL) p-tolualdehyde, 2mL 2-methylfuran, 0.1mmol sulfamic acid into a round bottom flask, then heat to 80°C with an oil bath under magnetic stirring, and react for 6 hours. Remove the oil bath and cool down to room temperature; the catalyst is filtered out, the mother liquor is concentrated by a rotary evaporator, and the residue is separated by silica gel thin-layer chromatography using petroleum ether as a developing solvent. Pure triarylmethane was obtained with an isolated yield of 98%. The nuclear magnetic analysis data of this compound are as follows:
[0021] 1 H NMR (400MHz, CDCl 3 )δ:7.15-7.09(m,4H),5.86-5.84(m,4H),5.29(s,1H),2.30(s,3H),2.22(s,6H). 13 C NMR (100MHz, CDCl 3 )δ: 153.0, 151.3, 137.0, 136.4, 129.1, 128.2, 108.0, 106.0, 44.7, 21.0, 13.6. MS: m / z 266.10[M] + ..
Embodiment 3
[0022] Embodiment 3.5, the synthesis of 5'-(p-bromobenzyl) bi-(2-methylfuran):
[0023] Add 1mmol (0.103mL) of p-bromobenzaldehyde, 2mL of 2-methylfuran, and 0.1mmol of sulfamic acid into a round-bottomed flask, then heat to 90°C with an oil bath under magnetic stirring, and react for 6 hours. Remove the oil bath and cool down to room temperature; the catalyst is filtered out, the mother liquor is concentrated by a rotary evaporator, and the residue is separated by silica gel thin-layer chromatography using petroleum ether as a developing solvent. Pure triarylmethane was obtained with an isolated yield of 91.2%. The nuclear magnetic analysis data of this compound are as follows:
[0024] 1 H NMR (400MHz, CDCl 3 )δ: 7.41(d, J=8.4Hz, 2H), 7.11(d, J=8.4Hz, 2H), 5.87-5.86(m, 4H), 5.28(s, 1H), 2.23(s, 6H). 13 C NMR (100MHz, CDCl 3 )δ: 152.1, 151.6, 139.0, 131.5, 130.1, 120.8, 108.3, 106.1, 44.5, 13.6. MS: m / z 330.00, 332.00 [M] + ..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com