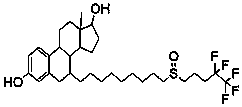
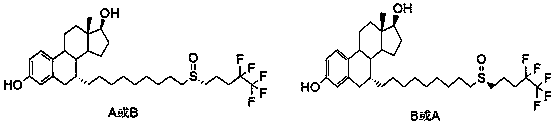
Improved method for recovering fulvestrant with unqualified isomer ratio
A technology for fulvestrant and isomers, which is applied in the field of pharmaceutical preparation, can solve the problems of unqualified fulvestrant recovery isomer ratio, unindustrialization, potential safety hazards, etc., so as to reduce production costs, improve recovery, The effect of reducing the discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
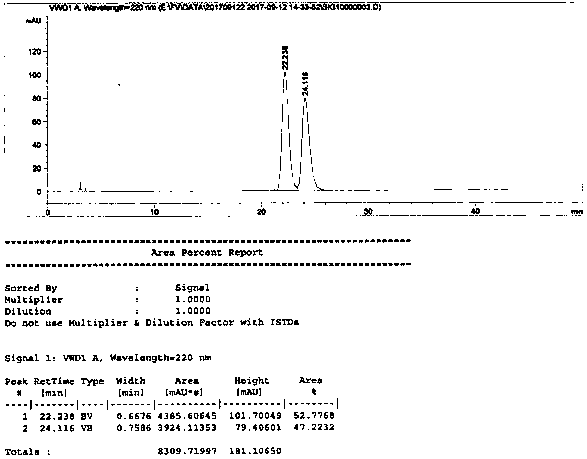
[0021] (1) Reduction reaction
[0022] Add 5 g of fulvestrant with an unqualified isomer ratio, 2.1 g of sodium bromide, 3.5 g of p-toluenesulfonic acid and 25 ml of acetone into a 100 ml three-necked flask, stir and react at 25°C-35°C for 1.5h, and the reaction solution gradually changes from After the clarification became viscous, the reaction was completed, filtered under reduced pressure, and the filtrate was concentrated under reduced pressure to obtain 4.6 g of light yellow oil.
[0023] (2) Oxidation reaction
[0024] Add 4.6g of the reduction product from the previous step into a 50ml three-necked flask, add 10.1ml of ethyl acetate and 2.63ml of acetic acid, slowly add 4.66ml of peracetic acid dropwise, keep the temperature below 25°C, and keep warm at 20°C after the addition The reaction was stirred at 25°C for 2 hours. After the reaction was completed, 25% aqueous solution of sodium thiosulfate was slowly added dropwise. Extract with 100ml of ethyl acetate, separa...
Embodiment 2
[0026] (1) Reduction reaction
[0027] Add 5g of fulvestrant with an unqualified isomer ratio, 3g NaI and 25ml of acetone into a 100ml three-necked flask, slowly add 25ml of 40% boron trifluoride ether solution dropwise, and stir and react at 25°C-35°C for 1.5h. After completion, add 30ml of toluene and 30ml of water, stir for 5min, stand still to separate the water layer, wash the toluene layer with 30ml of water, repeat once. The toluene layer was concentrated under reduced pressure at 50°C to obtain 4.4 g of light yellow oil.
[0028] (2) Oxidation reaction
[0029] Add 4.4g of the reduction product from the previous reaction to a 250ml three-necked flask, and add 44.5ml of tetrahydrofuran and 11.1ml of methanol; cool down to 0°C in an ice-salt water bath, add 4.3ml of peracetic acid, and the temperature does not exceed 5°C; the reaction solution is cloudy after adding , keep warm at 20°C-30°C for 20 hours; add 14.1ml of dichloromethane, wash the dichloromethane layer 4 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com