Method for preparing camptothecin prodrug double drug-loaded supramolecular hydrogel for drug combination
A supramolecular hydrogel, combined drug technology, applied in the field of biomedicine, can solve problems such as difficulty in achieving targeted drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
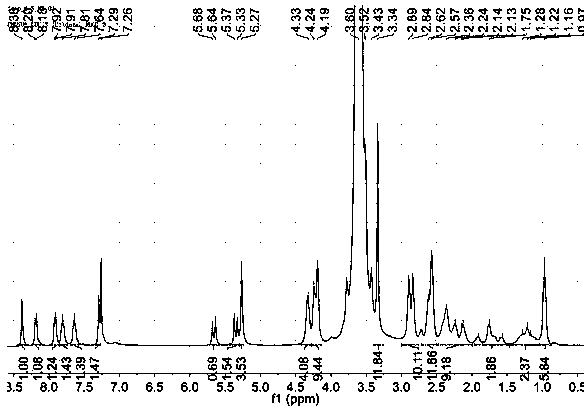
[0056] Embodiment 1: the preparation of camptothecin prodrug
[0057] (1) Synthesis of mPEG-COOH: In a 50 mL round bottom flask, weigh mPEG (10.0 g, 5 mmol, Mw=1900) and succinic anhydride (1.0 g, 10 mmol), add 25 mL of anhydrous dichloromethane , stirred to dissolve, then slowly added pyridine (0.8 mL, 10 mmol) dropwise, after the dropwise addition, refluxed for 3 days under the protection of nitrogen. The reaction solution was concentrated under reduced pressure and recrystallized to obtain mPEG-COOH (8.9 g, yield: 88%).
[0058] (2) Preparation of reductively responsive camptothecin prodrug monomer CPT-SS: After adding CPT (2 g, 5.74 mmol) into a 150 mL round bottom flask, add 75 mL of dichloromethane and stir until cloudy, then add DMAP (2.11g, 17.3 mmol), added triphosgene (0.567 g, 1.92 mmol) after ice bath for 5 min, stirred in ice bath for 2 h, then added dropwise to the above reaction solution containing bis(2-hydroxyethyl) disulfide ( 90%) in tetrahydrofuran (1.77 ...
Embodiment 2
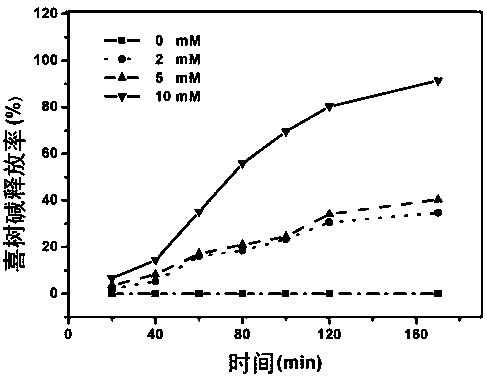
[0061] Example 2, preparation of camptothecin prodrug supramolecular hydrogel loaded with 5-fluorouracil
[0062] Dissolve CPT-S-S-mPEG (20 mg, 8 µmol) in 1 ml of water to form a uniform aqueous solution; take water-soluble anticancer drug 5-FU (5 mg, 38 µmol) and add it to the aqueous solution of CPT-S-S-mPEG, Ultrasonic dissolution at room temperature; take α-CD (100 mg, 0.1 mmol), add it to the mixed aqueous solution of CPT-S-S-mPEG and 5-FU, ultrasonically disperse at room temperature for 5 min, and let it stand for 1 min to form a supramolecular hydrogel. The pH value of the hydrogel is 6~8.
Embodiment 3
[0063] Example 3, preparation of camptothecin prodrug supramolecular hydrogel loaded with cisplatin
[0064] Dissolve CPT-S-S-mPEG (20 mg, 8 µmol) in 1 ml of water to form a uniform aqueous solution; take water-soluble anticancer drug cisplatin (3 mg, 10 µmol) and add it to the aqueous solution of CPT-S-S-mPEG, Ultrasonic dissolution at room temperature, take α-CD (100 mg, 0.1 mmol), add it to the mixed aqueous solution of CPT-S-S-mPEG and cisplatin, ultrasonically disperse at room temperature for 5 min, and let it stand for 30 min to form a supramolecular hydrogel. The pH value of the hydrogel is 6-8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com