Purifying method of tacrolimus 8-epimer
A technology of epimers and tacrolimus, which is applied in the field of biopharmaceuticals, can solve problems such as separation and purification of tacrolimus, and achieve the effect of reducing adverse reactions and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
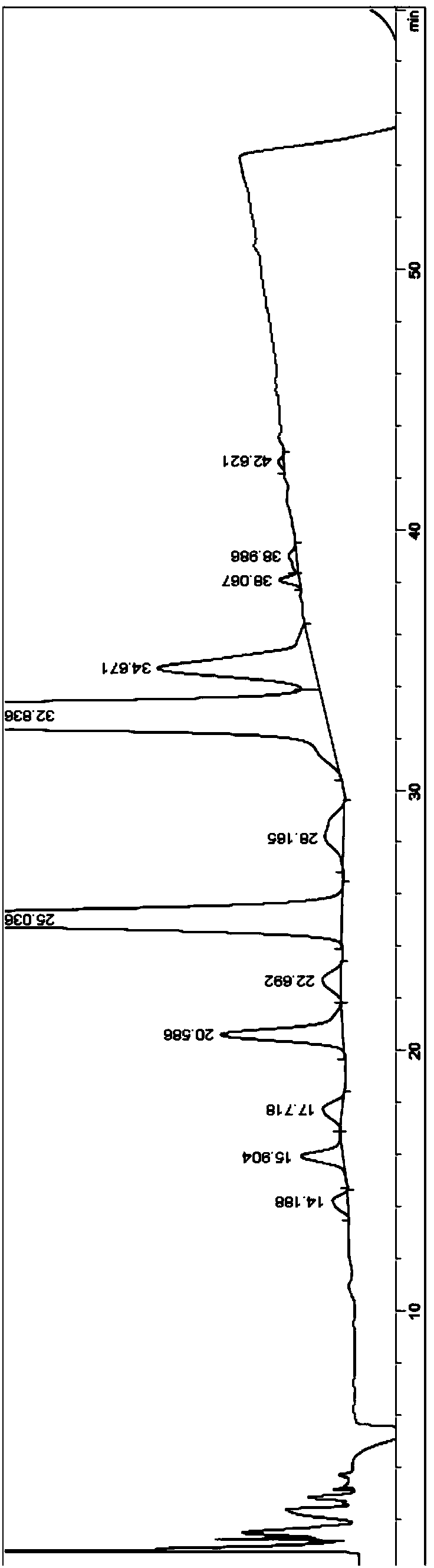
[0034] 30 g of tacrolimus raw material was dissolved in 300 ml of acetonitrile, filtered through a filter membrane with a filter pore size of 0.22 μm, and prepared into a 100 mg / ml tacrolimus solution (ie, a sample solution). In an embodiment, the filter membrane is a ceramic membrane. Then, use a liquid chromatography column to separate and purify the sample solution to obtain a purified solution.
[0035] The chromatographic conditions of the liquid chromatography column are as follows:
[0036]
[0037] The impurity peak behind the tacrolimus peak was collected, and the collected solution was concentrated at a concentration temperature of 30°C and a crystallization temperature of 5°C.
[0038] The crystallization solution was filtered, and the crude product of tacrolimus 8-epimer was obtained by filtration. The crude product of tacrolimus 8-epimer was dissolved in acetonitrile, and filtered with a filter membrane with a filter pore size of 0.22 μm, which was a ceramic ...
Embodiment 2
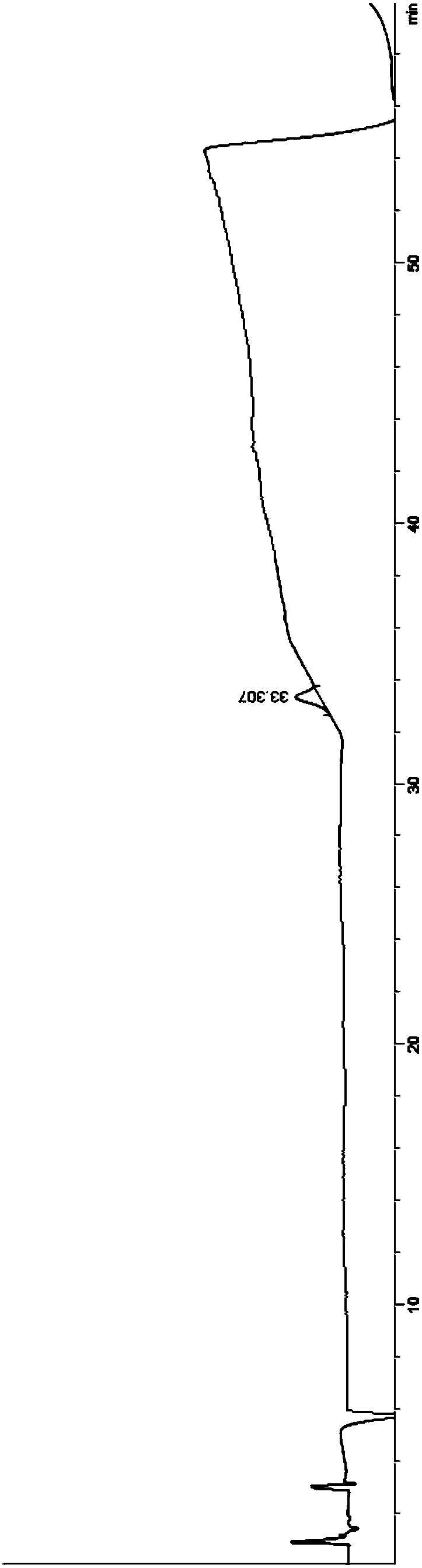
[0041] 60 g of tacrolimus raw material was dissolved in 300 ml of acetonitrile, filtered through a filter membrane with a filter pore size of 0.22 μm, and prepared into a 200 mg / ml tacrolimus solution (ie, a sample solution). In the embodiment, the filter membrane is selected from polytetrafluoroethylene membrane. Then, use a liquid chromatography column to separate and purify the sample solution to obtain a purified solution.
[0042] The chromatographic conditions of the liquid chromatography column are as follows:
[0043]
[0044] The impurity peak behind the tacrolimus peak was collected, and the collected solution was concentrated at a concentration temperature of 40°C and a crystallization temperature of 10°C.
[0045] The crystallization solution was filtered, and the crude product of tacrolimus 8-epimer was obtained by filtration. The crude tacrolimus 8-epimer was dissolved in acetonitrile, filtered through a filter membrane with a pore size of 0.22 μm, and the f...
Embodiment 3
[0047] 60 g of the tacrolimus raw material was dissolved in 300 ml of methanol, filtered through a filter membrane with a filter pore size of 0.22 μm, and prepared into a 200 mg / ml tacrolimus solution (ie, a sample solution). In an embodiment, the filter membrane is a mixed fiber microporous filter membrane. Then, use a liquid chromatography column to separate and purify the sample solution to obtain a purified solution.
[0048] The chromatographic conditions of the liquid chromatography column are as follows:
[0049]
[0050] The impurity peak behind the tacrolimus peak was collected, and the collected solution was concentrated at a concentration temperature of 40°C and a crystallization temperature of 10°C.
[0051]The crystallization solution was filtered, and the crude product of tacrolimus 8-epimer was obtained by filtration. The crude product of tacrolimus 8-epimer was dissolved in methanol, filtered through a filter membrane with a filter pore size of 0.22 μm (sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com