Synthetic method for anti-HIV active sulfonic acid based bagasse xylan p-ferrocene benzoate
A technology of benzoate and xylan, which is applied in the direction of antiviral agents, can solve the problems of limited anti-HIV activity, and achieve the effect of stable product quality and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
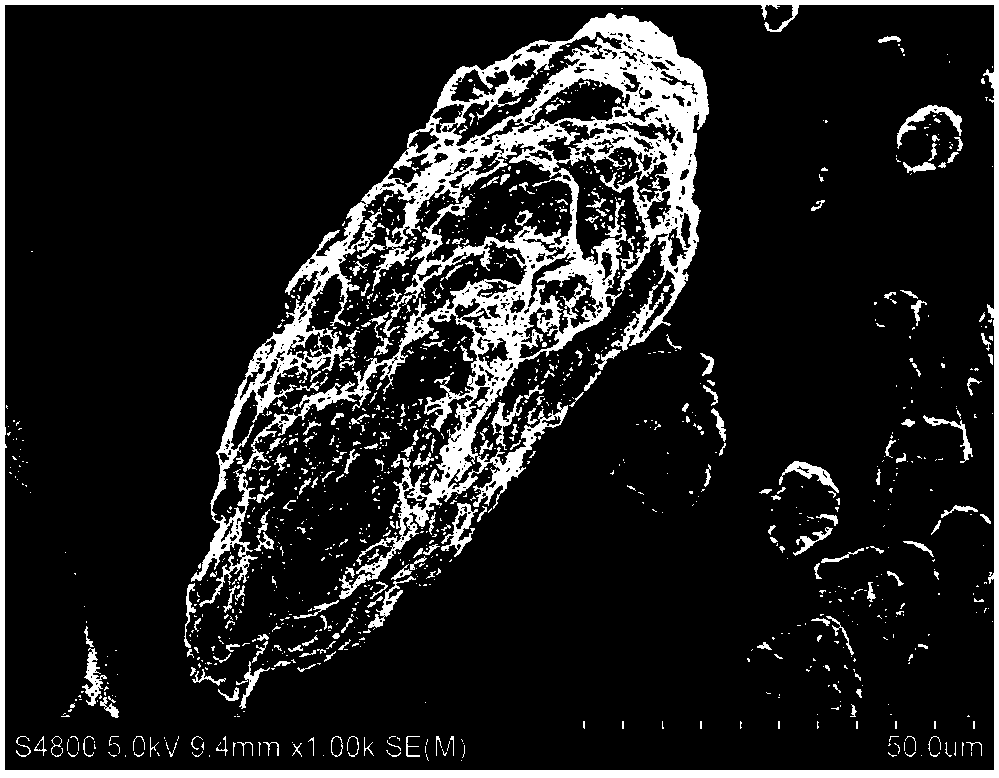
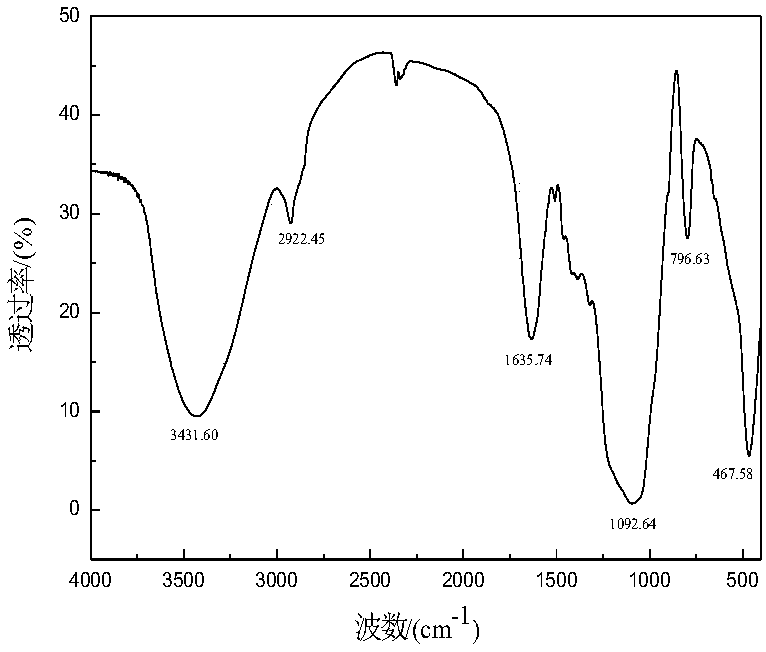
Image
Examples
Embodiment
[0046] (1) Drying 10 g of bagasse xylan in a vacuum constant temperature drying oven at 60°C for 24 hours to constant weight to obtain dry bagasse xylan.
[0047] (2) 8g solid NaHSO 3 Add 30mL of distilled water into a 250mL four-necked flask in turn, stir and dissolve at room temperature to obtain a mass fraction of 21% NaHSO 3 solution.
[0048] (3) heating the NaHSO with a mass fraction of 21% obtained in step (2) under stirring 3 The solution was brought to 85°C, and 20% NaNO was added dropwise. 2 7 mL of the solution was added dropwise under control for 20 minutes, and the reaction was continued to stir for 15 minutes to obtain an esterification agent sodium sulfamate solution.
[0049] (4) Weigh 3 g of dry-based bagasse xylan obtained in step (1) and add it to the reaction system obtained in step (3), then add 0.03 g of catalyst 12-tungstophosphoric acid to it, control the reaction temperature to be 55°C, and add mass fraction It was 2 mL of 5% sodium hydroxide solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com