Method for Establishing Fingerprint of Bailemian Capsules
A technology of fingerprint spectrum and establishment method, which is applied to the establishment method of Bailemian Capsule fingerprint and the field of fingerprint spectrum, can solve the problem that the quality detection method of Bailemian Capsule cannot comprehensively reflect the quality of Bailemian Capsule as a whole.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 100
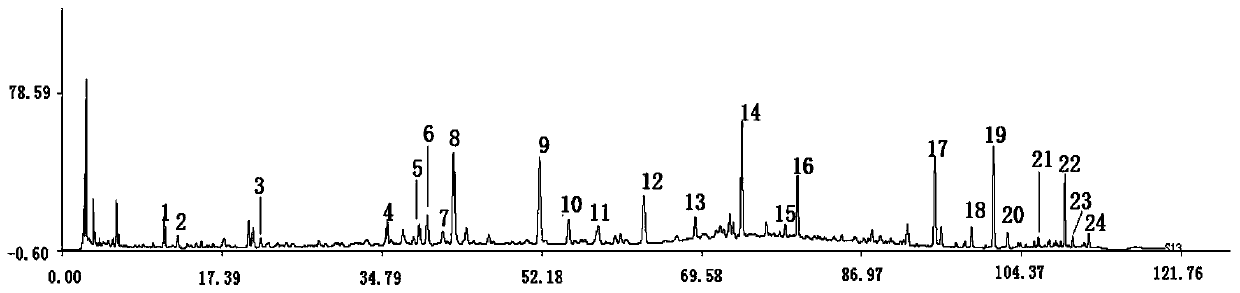
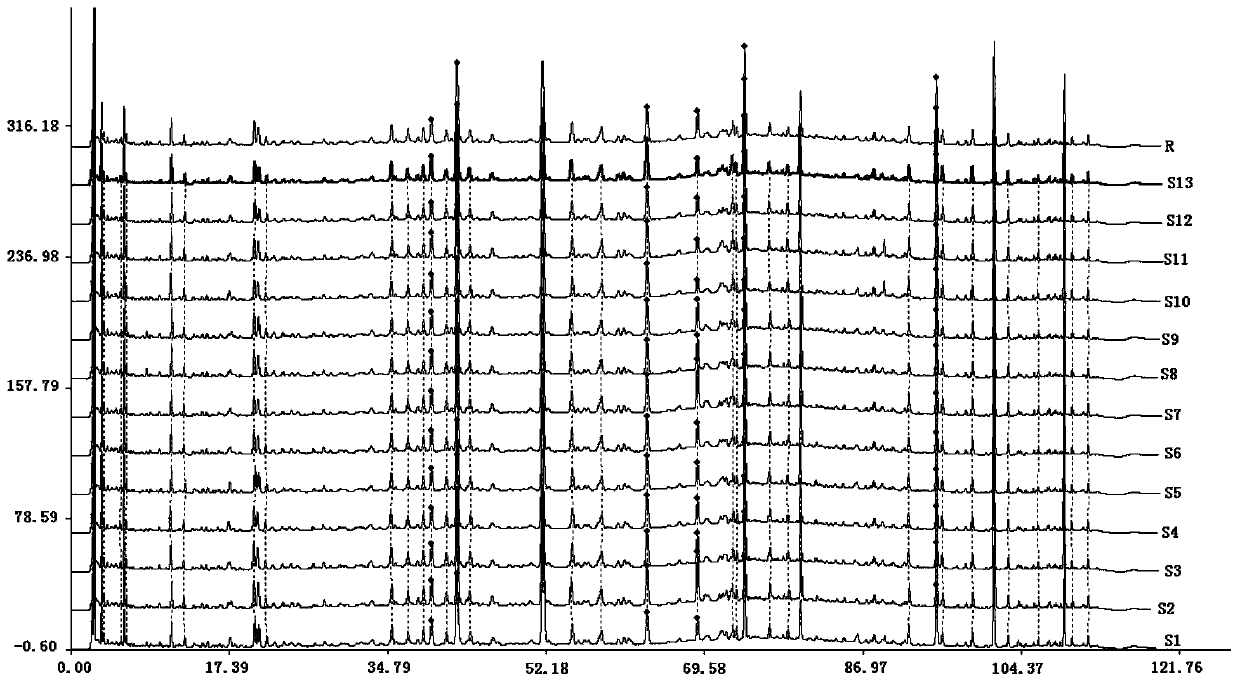
[0074] The method for establishing the fingerprint of Bailemian Capsules in this embodiment comprises the following steps:
[0075] (1) Preparation of the test solution: take 1.0 g of the contents of Bailemian Capsules, weigh them accurately, put them in a stoppered Erlenmeyer flask, add 25 mL of methanol aqueous solution with a volume fraction of 70%, weigh them, and heat to reflux for 30 minutes , let it cool, use a volume fraction of 70% methanol aqueous solution to make up the lost weight, shake well, pass through a 0.45 μm microporous membrane, and take the subsequent filtrate as the test solution;
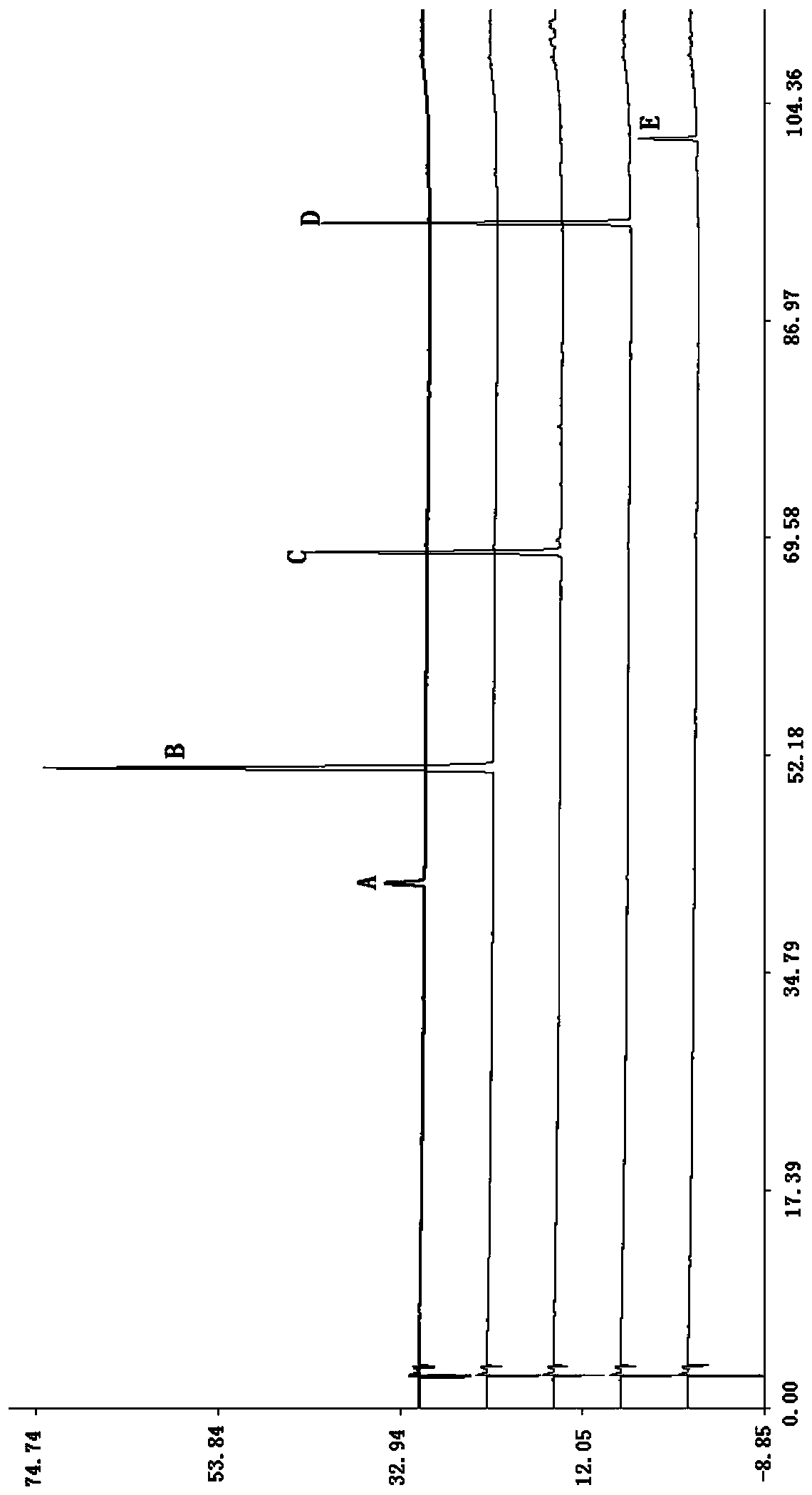
[0076] (2) Preparation of reference solution: accurately weigh quercitrin reference substance, 2,3,5,4′-tetrahydroxystilbene-2-O-D glucoside reference substance, schisandrol A reference substance, dandelion Phenolic acid B reference substance, emodin reference substance, add volume fraction of 70% methanol aqueous solution to make each 1mL containing 65μg quercitrin, 45μg 2,3...
Embodiment 2
[0081] The method for establishing the fingerprint of Bailemian Capsules in this embodiment comprises the following steps:
[0082] (1) Preparation of the test solution: take 1.0 g of the contents of Bailemian Capsules, weigh them accurately, put them in a stoppered Erlenmeyer flask, add 25 mL of methanol aqueous solution with a volume fraction of 70%, weigh them, and heat to reflux for 60 minutes , let it cool, use a volume fraction of 70% methanol aqueous solution to make up the lost weight, shake well, pass through a 0.45 μm microporous membrane, and take the subsequent filtrate as the test solution;
[0083] (2) Preparation of reference solution: accurately weigh quercetin reference substance, 2,3,5,4′-tetrahydroxystilbene-2-O-D glucoside reference substance, schisandra alcohol A reference substance, salvianol Acid B reference substance, emodin reference substance, add volume fraction of 70% methanol aqueous solution to make each 1mL containing 65μg quercitrin and 45μg 2,3...
Embodiment 3
[0088] (1) Preparation of the test solution: take 1.0 g of the contents of Bailemian Capsules, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 25 mL of methanol precisely, weigh it, heat to reflux for 90 minutes, let it cool, and use methanol Make up for the lost weight, shake well, pass through a 0.45 μm microporous membrane, and take the subsequent filtrate as the test solution;
[0089] (2) Preparation of reference solution: accurately weigh quercetin reference substance, 2,3,5,4′-tetrahydroxystilbene-2-O-D glucoside reference substance, schisandra alcohol A reference substance, salvianol Acid B reference substance and emodin reference substance were prepared by adding methanol, each containing 65 μg quercitrin, 45 μg 2,3,5,4′-tetrahydroxystilbene-2-O-D glucoside, 48 μg schisandrin A, 72 μg salvianolic acid B, 4 μg emodin reference substance solution;
[0090] (3) Determination of HPLC chromatographic conditions: the chromatographic column uses octadecylsi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com