A Construction Method and Application of Fingerprint of Shenzhiling Oral Liquid
A Shenzhiling oral liquid and fingerprint technology, which is applied in the construction and application field of the fingerprint of Shenzhiling oral liquid, can solve the problems of limited resolution and peak capacity, achieve improved separation selectivity, good reproducibility, The effect of high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] (1) Preparation of the test solution: take Shenzhiling Oral Liquid, add methanol, ultrasonically extract, refrigerate and place for 0.5-1 hour, return to room temperature, filter, and take the filtrate to obtain the test solution;
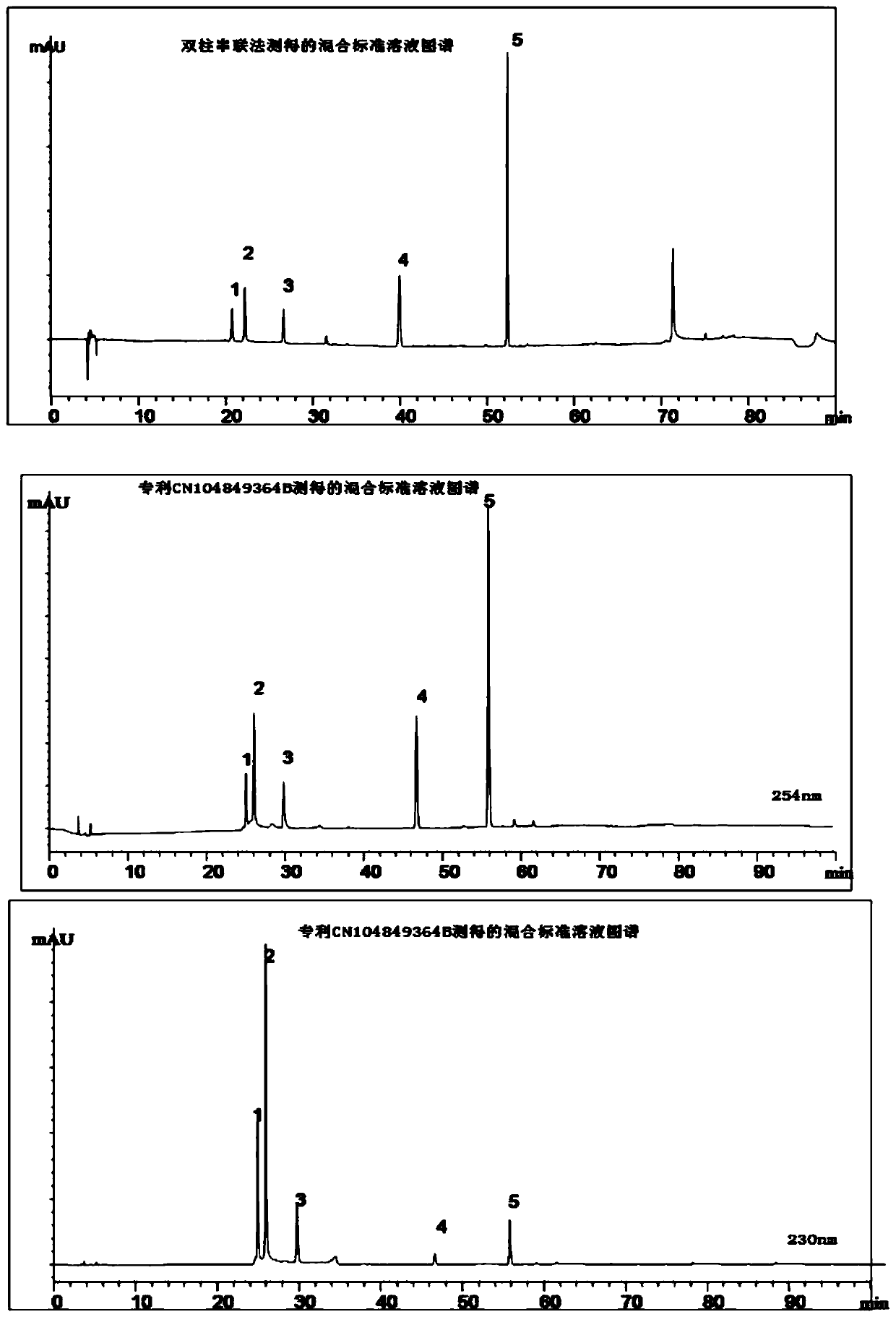
[0039] (2) Preparation of reference substance solution: take paeonifloride glucoside, paeoniflorin, liquiritin, cinnamic acid, and ammonium glycyrrhizinate reference substances dried under reduced pressure to constant weight, and add methanol respectively to prepare reference substance solutions;
[0040] (3) Determination: Accurately draw need testing solution and reference substance solution respectively, inject high performance liquid chromatograph and detect through HPLC separation, the composition of mobile phase is acetonitrile-0.1% phosphoric acid aqueous solution, adopts gradient elution, and chromatographic column is by C1( TMS) in series with C18 to form, and use fingerprint software to analyze and process the spectrum obtained by h...
Embodiment 1
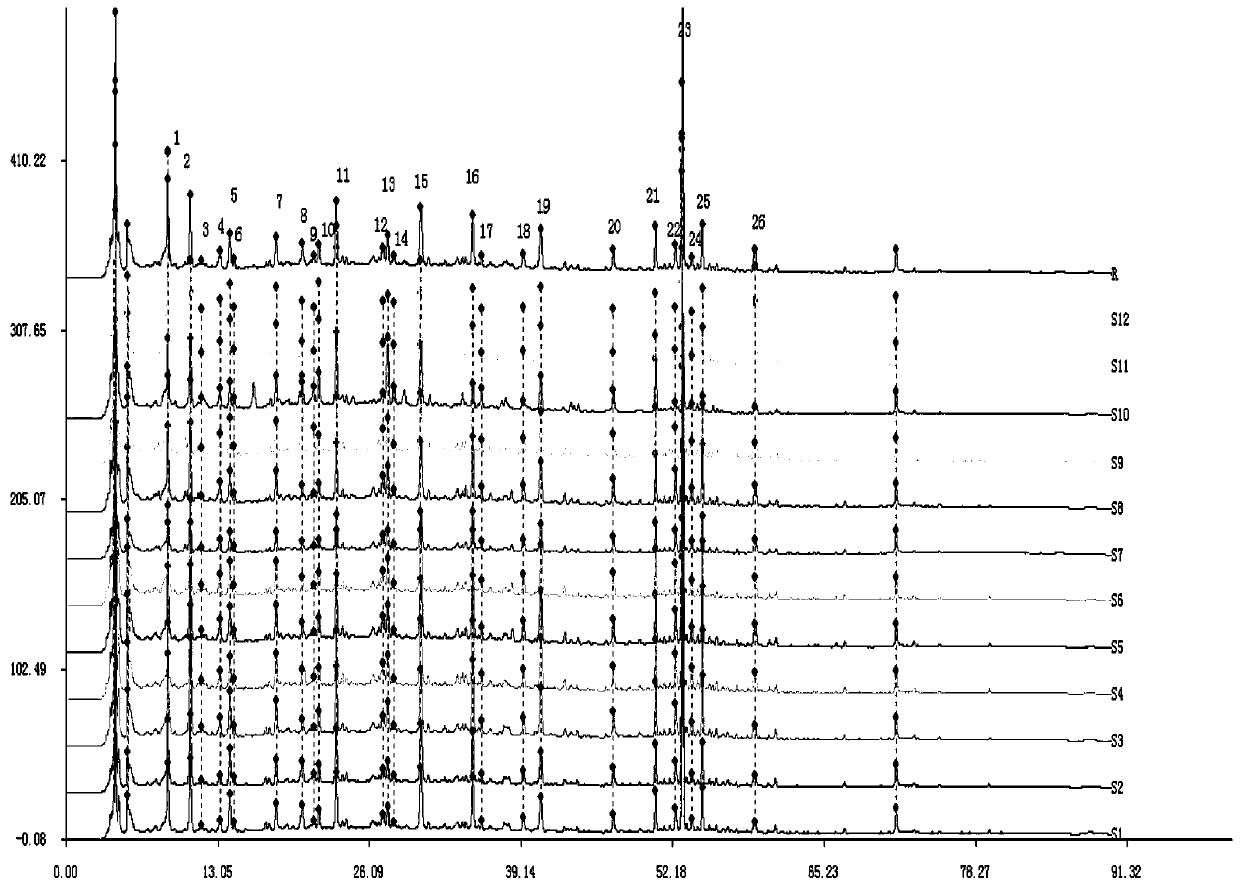
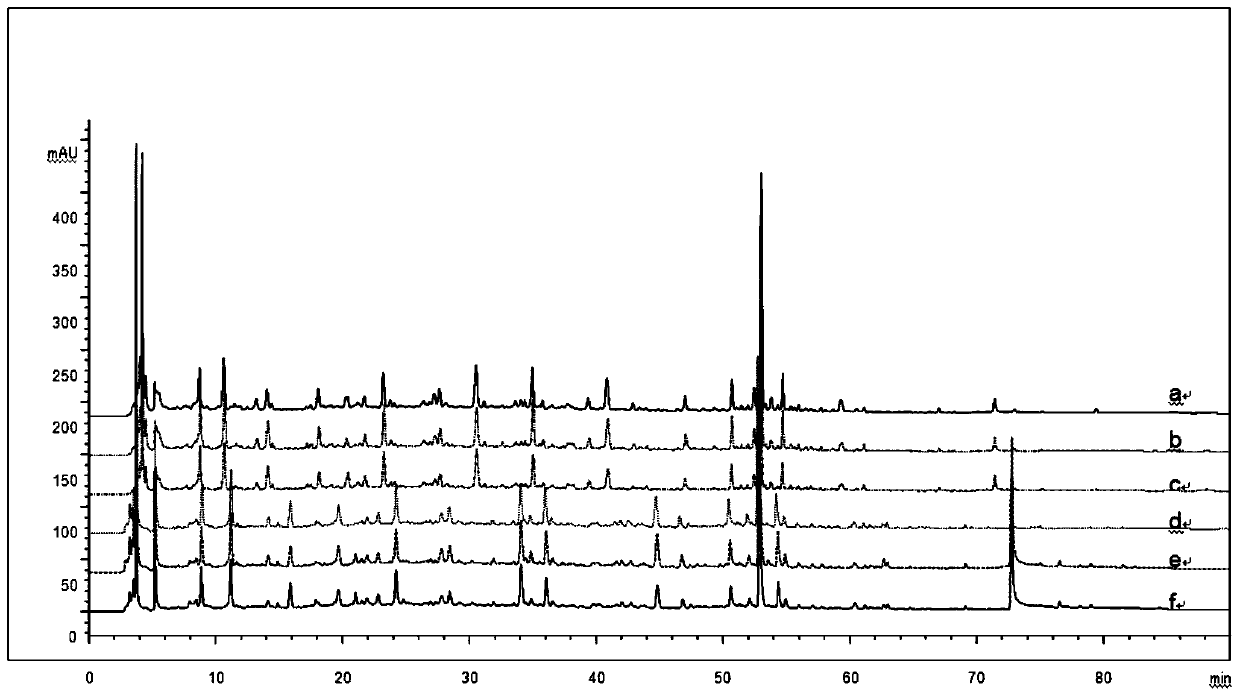
[0059] Example 1: Construction and application of the fingerprint of Shenzhiling Oral Liquid based on double-column series connection
[0060] The chromatographic conditions are: the chromatographic column is YMC-Pack TMS (5μm, 100×4.6mm) in series with JADE-PAK ODS-AQ chromatographic column (5μm, 150×4.6mm); mobile phase: mobile phase A is 0.1% phosphoric acid aqueous solution, mobile Phase B is acetonitrile, elution method: gradient elution; flow rate 0.8ml / min, column temperature 30°C, detection wavelength 254nm, injection volume 10μl, theoretical plate number not less than 8000 based on the chromatographic peak of cinnamic acid, all groups The scores were tested within 90 minutes. Wherein, the mobile phase linear gradient is shown in Table 1:
[0061] Table 1 Gradient elution conditions of chromatographic mobile phase
[0062]
[0063] (1) Preparation of the test solution: Precisely measure 1.25ml of Shenzhiling Oral Liquid in a 25ml volumetric flask, add methanol to ...
Embodiment example 2
[0072] Implementation Case 2: Methodological Investigation
[0073] The methodological investigation mainly examined the precision, stability and repeatability. The inspection methods and results are as follows:
[0074] (1) Precision test The same batch of Shenzhiling Oral Liquid samples were taken. After pretreatment, under the optimal chromatographic conditions, the two-column direct series method was used to continuously measure 6 times and record the chromatograms. The results are shown in Table 3. It can be seen from the table that the relative retention time ratio of each main chromatographic peak has no obvious change, and the RSD is 0.037%-0.31%. Except for No. 14 chromatographic peak, the relative peak area RSD of each main chromatographic peak is 0.7%-4.2%, RSD<5.0%, indicating that the precision of the instrument is good. Because the common peak area of No. 14 is small (<1% of the total area), its RSD value is relatively large.
[0075] (2) Stability test The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com