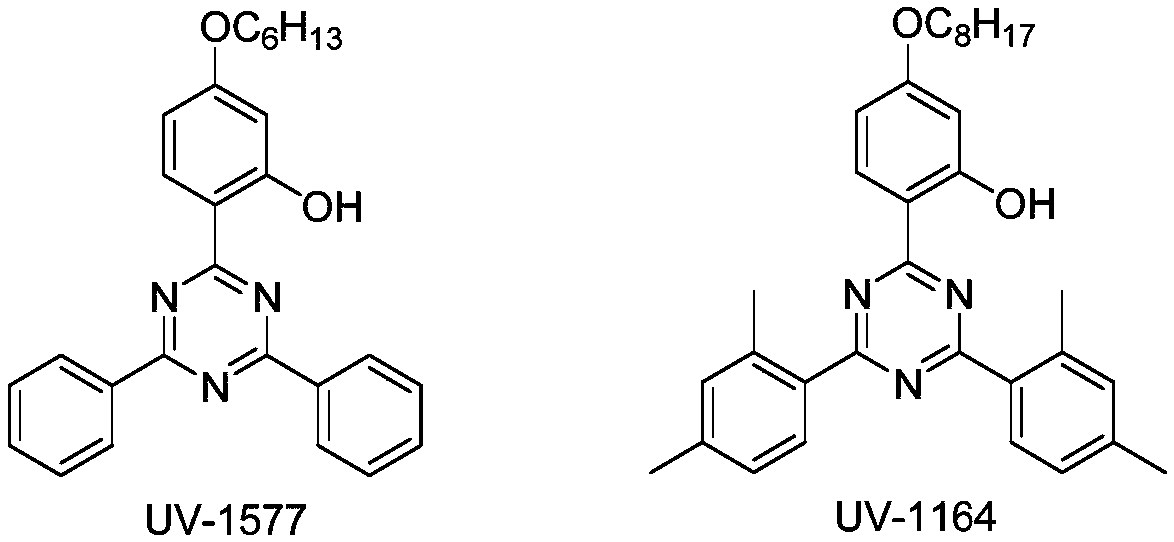
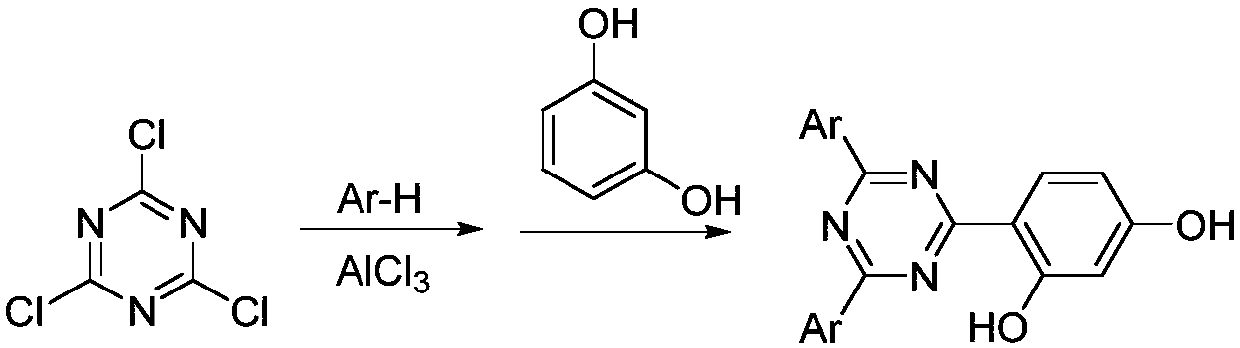
Method of recycling catalyst used in Friedel-Crafts process for producing aryl-s-triazine ultraviolet absorber
A s-triazine, ultraviolet light technology, applied in the direction of organic chemistry, can solve the problems of high cost, aluminum trichloride can not be recycled, pollute the environment, etc., to improve the level of clean production, eliminate aluminum trichloride-containing wastewater Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 3 liters of chlorobenzene, 184 grams of cyanuric chloride, 156 grams of benzene (2 times the amount) and 400 grams of aluminum chloride (3 times the amount) in 5 liters of there-necked flasks equipped with mechanical stirring; Add 20 milliliters of concentrated hydrochloric acid (0.2 times), stir and react for 1 hour, rise to room temperature and then heat to 90°C, add 1.2 kilograms of 1-methyl-3-ethylimidazole tetrachloroaluminate ionic liquid (imidazole / aluminum chloride =1:2), stirred for half an hour. The upper layer is the chlorobenzene solution, and the lower layer is the ionic liquid layer. After liquid separation, the chlorobenzene layer recovers part of the solvent, filters the precipitated solid, washes with water, and recrystallizes to obtain monochlorodiphenyl-1,3,5-tri 215 grams (81%) of oxazine; 1 gram of aluminum trichloride seed was added to the ionic liquid layer, cooled to room temperature, stirred slowly overnight, and 220 grams of powdered alumin...
Embodiment 2
[0031] Add 3 liters of chlorobenzene, 184 grams of cyanuric chloride, 156 grams of benzene (2 times the amount) and 400 grams of aluminum chloride (3 times the amount) in 5 liters of there-necked flasks equipped with mechanical stirring; Add 20 ml of concentrated hydrochloric acid (0.2 times), stir the reaction for 1 hour, rise to room temperature and add 121 g of resorcinol (1.1 times), then heat to 90° C. for 2 hours. After the reaction, add 1.2 kg of 1-methyl-3-ethylimidazole tetrachloroaluminate ionic liquid (imidazole / aluminum chloride = 1:2), stir for half an hour, then filter while hot, and filter the cake with 0.2 liter of chlorine Wash with benzene, and recrystallize the solid to obtain 228 grams of diphenyl-6-(1-resorcinyl)-1,3,5-triazine product (67% yield); add 1 gram of aluminum trichloride seed to the filtrate , cooled to room temperature, stirred slowly overnight, and filtered out 295 grams of powdery aluminum trichloride;
Embodiment 3
[0033] Add 3 liters of chlorobenzene, 184 grams of cyanuric chloride, 212 grams of m-xylene (2 times the amount) and 400 grams of aluminum chloride (3 times the amount) in a 5 liters there-necked flask equipped with mechanical stirring; ice water cooling Next, add 20 milliliters of concentrated hydrochloric acid (0.2 times), stir and react for 1 hour, rise to room temperature and heat to 90 ° C, then add 1.2 kilograms of 1-methyl-3-ethylimidazole tetrachloroaluminate ionic liquid (imidazole / aluminum chloride=1:2), stirred for half an hour. The upper layer is the chlorobenzene solution, and the lower layer is the ionic liquid layer. After liquid separation, the chlorobenzene layer recovers part of the solvent, and the solid is filtered to recrystallize to obtain the product monochloro-2,4-(1-m-xylyl)- 308 grams (95%) of 1,3,5-triazine; 1 gram of aluminum trichloride seed was added to the ionic liquid layer, stirred slowly at room temperature overnight, and 226 grams of precipi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com