Synthesis process of 1,4-cyclohexane diisooctoate
A cyclohexanedimethanol and synthesis process technology, which is applied in the field of synthetic esters, can solve the problems of environmental pollution, complex process routes, and high production costs, and achieve the effects of green and environmentally friendly process routes, simple synthetic routes, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for synthesizing 1,4-cyclohexanedimethanol diisooctanoate, the preparation steps include.
[0025] Step 1, the synthesis of 1,4-cyclohexanedimethanol.
[0026] 1. Add 50 mol of terephthalic acid and 50 mol of (4-methylcyclohexyl)methanol into the reaction kettle under superatmospheric pressure and temperature of 250-400°C, stir well, and fully react to obtain terephthalic acid bis (4-Methylcyclohexyl)methyl ester.
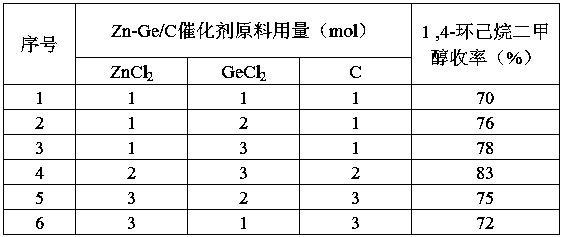
[0027] 2. Add Zn-Ge / C catalyst to the reaction system, feed 100 mol of hydrogen, and fill in hydrogen for hydrogenation reaction. During the hydrogenation reaction, control the hydrogenation rate to keep the temperature in the reactor at 150°C and the pressure at 3Mpa, after the hydrogenation reaction, cool to 90°C, slowly open the exhaust port to obtain 1,4-cyclohexanedimethanol.
[0028] Step 2, the synthesis of 1,4-cyclohexanedimethanol diisocaprylate.
[0029] 1. Add the 1,4-cyclohexanedimethanol and isooctanoic acid prepared in step 1 into t...
Embodiment 2
[0033] A method for synthesizing 1,4-cyclohexanedimethanol diisooctanoate, the preparation steps include.
[0034] Step 1, the synthesis of 1,4-cyclohexanedimethanol.
[0035] 1. Add 70 mol of terephthalic acid and 70 mol of (4-methylcyclohexyl)methanol into the reaction kettle under superatmospheric pressure and a temperature of 400°C. After stirring evenly, fully react to obtain terephthalic acid bis(4 -methylcyclohexyl) methyl ester.
[0036] 2. Add Zn-Ge / C catalyst to the reaction system, feed 140mol of hydrogen, and fill in hydrogen for hydrogenation reaction. During the hydrogenation reaction, control the hydrogenation rate to keep the temperature in the reactor at 180°C and the pressure at 1Mpa, after the hydrogenation reaction, cool to 100°C, slowly open the exhaust port to obtain 1,4-cyclohexanedimethanol.
[0037] Step 2, the synthesis of 1,4-cyclohexanedimethanol diisocaprylate.
[0038] 1. Add the 1,4-cyclohexanedimethanol and isooctanoic acid prepared in step 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com