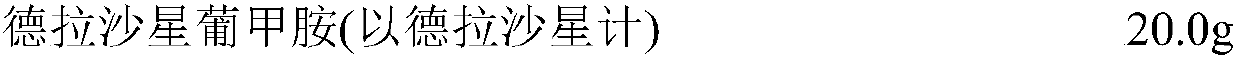Delafloxacin meglumine freeze-dried preparation used for injection, and preparation method thereof
A technology of delafloxacin meglumine and freeze-dried preparations, which is applied in the field of medicine, can solve the problems of hidden safety hazards for patients, less excipient cyclodextrin, toxic and side effects, etc., and achieve stable storage quality, improved safety, and resolubility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] prescription
[0022]
[0023]
[0024] Preparation Process
[0025] Weigh the prescribed amount of absolute ethanol and polyethylene glycol 15-hydroxystearate, add it to about 700ml of water for injection pre-cooled to room temperature, stir the solution to become a colorless clear liquid, add the prescribed amount of delafloxacin glucagon Stir amine and glucagon until completely dissolved, adjust the pH value to 6.0 with 1M hydrochloric acid solution, adjust the volume to the total amount, stir evenly; filter, fill, freeze-dry, and visually inspect to obtain the finished product.
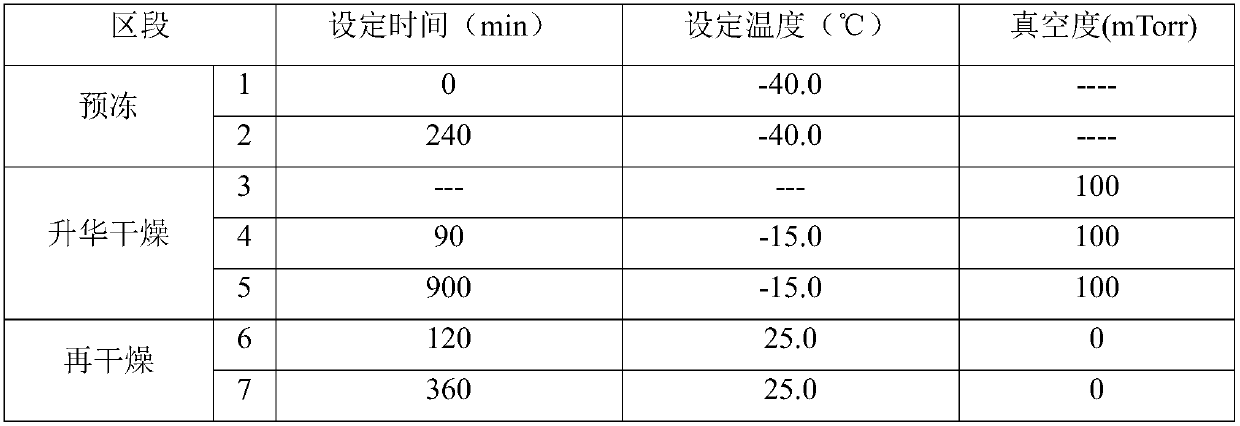
[0026] Freeze-drying curve:
[0027]
Embodiment 2
[0029] prescription
[0030]
[0031] Preparation method (with embodiment 1)
Embodiment 3
[0033] prescription
[0034]
[0035]
[0036] Preparation method (with embodiment 1)
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com