Electrolyte and lithium ion battery
A technology of electrolyte and lithium salt, applied in the field of electrolyte and lithium ion battery, can solve the problems of oxidative decomposition of electrolyte solvent, deterioration of battery cycle performance, unsatisfactory high-voltage resistance performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a preparation method of electrolyte, comprising:
[0035] Mix the solvent and lithium salt to obtain an electrolyte; the solvent is selected from one or more of fluorocarbonate solvents, sulfone solvents and nitrile solvents; the concentration of the lithium salt is 3.1-5.0mol / L .
[0036] In the present invention, the solvent and the lithium salt are mixed to obtain an electrolyte solution. The present invention does not limit the mixing method, and the mixing method well-known to those skilled in the art is sufficient.
[0037] The solvent and lithium salt have been clearly described above, and will not be repeated here.
[0038]The present invention provides a lithium ion battery, comprising the electrolyte described in the above technical solution.
[0039] In the present invention, the battery includes a positive electrode, a negative electrode, a diaphragm, and an electrolyte, and the electrolyte is the electrolyte described in the above t...
Embodiment 1
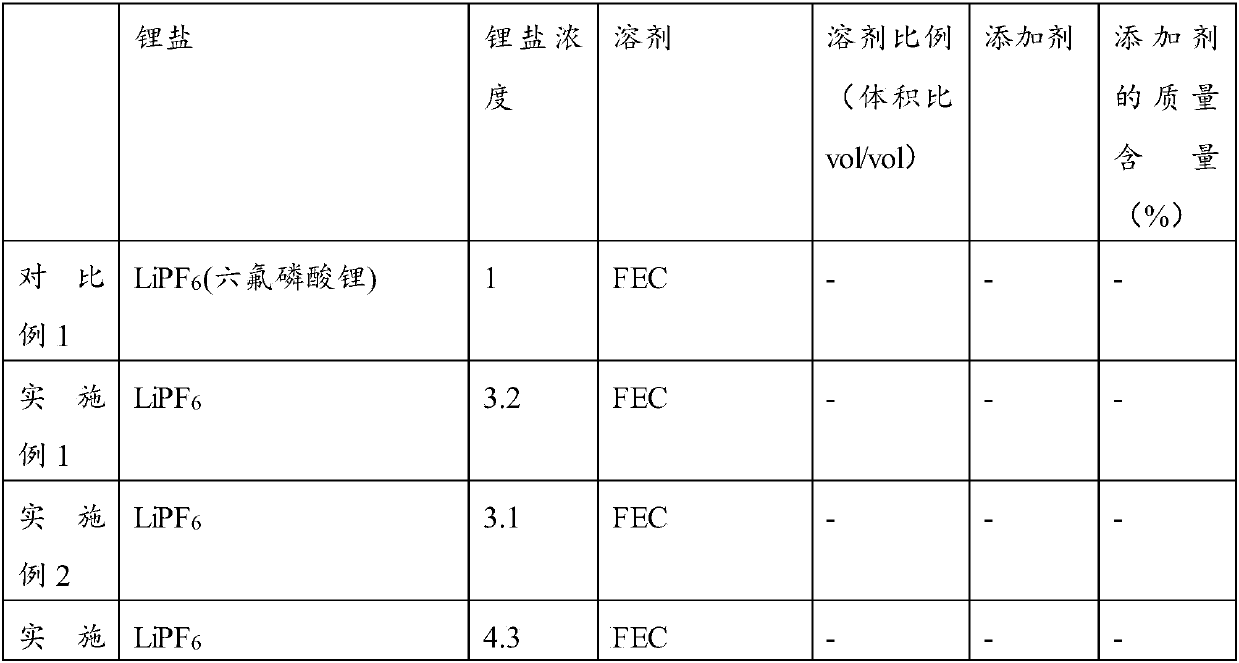
[0044] In a glove box filled with argon, weigh 15ml of fluoroethylene carbonate (FEC) and slowly add lithium salt LiPF 6 , so that the concentration of the lithium salt is 3.3mol / L, stirring until the lithium salt is completely dissolved to obtain an electrolyte.
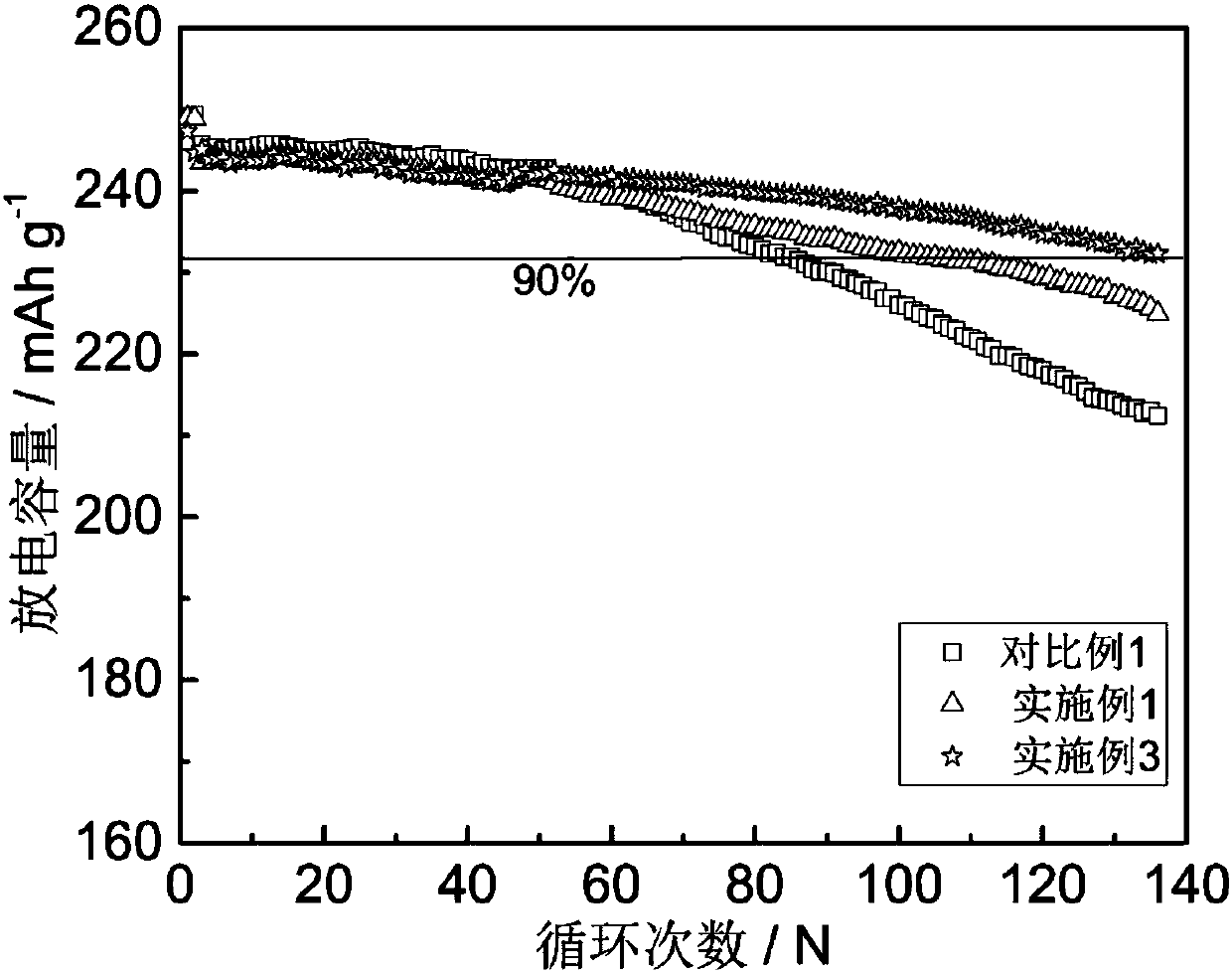
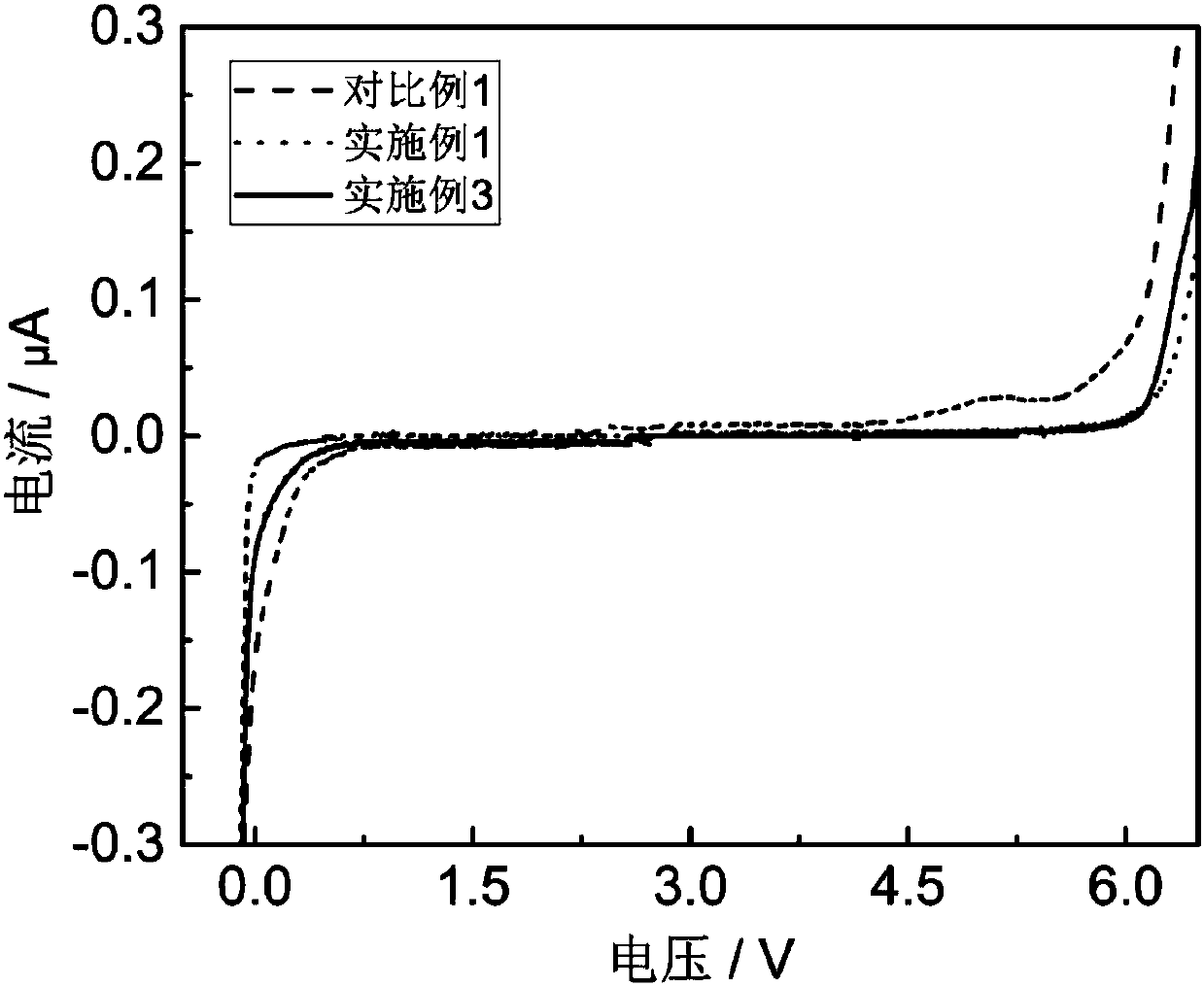
[0045] Performance test: put 2mL of the electrolyte prepared above into a conductivity tester to measure its conductivity. The electrochemical window was measured with 3mL electrolyte. Use Li 1.16 [Mn 0.75 Ni 0.25 ] 0.84 o 2 (High-voltage positive electrode) is a button battery with positive electrode material and pure metal Li as negative electrode material. The test voltage range is 2.6-4.8V, and the charge and discharge capacity of the battery is tested at 25°C. The cycle when the capacity decays to 90% of the initial capacity The number of times is 129 times.
Embodiment 2
[0050] In a glove box filled with argon, weigh 15ml of fluoroethylene carbonate (FEC) and slowly add lithium salt LiPF 6 , so that the concentration of the lithium salt is 3.1mol / L, stirring until the lithium salt is completely dissolved to obtain an electrolyte.
[0051] Performance test: put 2mL of the electrolyte prepared above into a conductivity tester to measure its conductivity. The electrochemical window was measured with 3mL electrolyte. Use Li 1.16 [Mn 0.75 Ni 0.25 ] 0.84 o 2 (High-voltage positive electrode) is a button battery with positive electrode material and pure metal Li as negative electrode material. The test voltage range is 2.6-4.8V, and the charge and discharge capacity of the battery is tested at 25°C. The cycle when the capacity decays to 90% of the initial capacity The number of times is 108 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com