Stable levocetirizine hydrochloride oral solution and preparation method of same
A technology of levocetirizine hydrochloride and oral solution, which is applied in the field of levocetirizine hydrochloride oral solution and its preparation, can solve problems such as toxicity and difficult to degrade, and achieve the effects of low toxicity, good stability and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
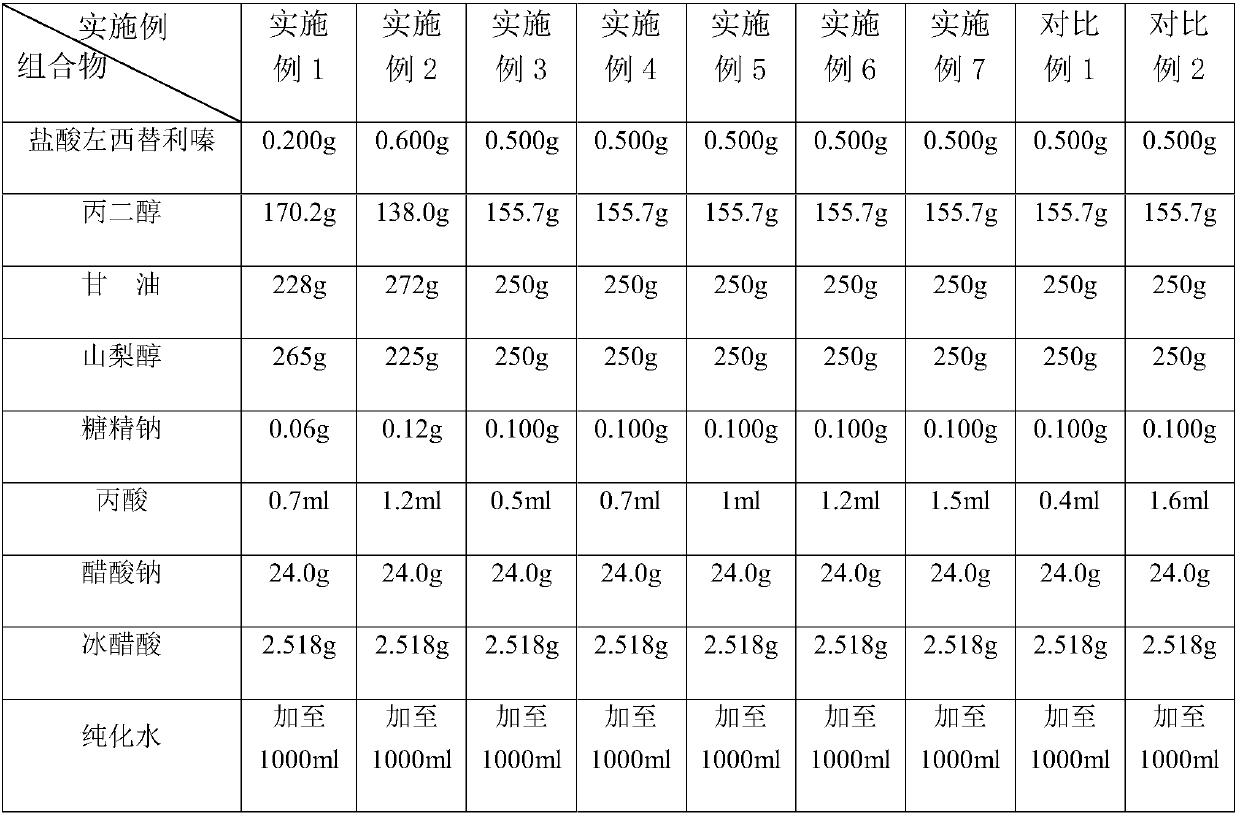
[0042] The preparation method of embodiment 8 levocetirizine hydrochloride oral solution
[0043] ① Dissolving sorbitol: Dissolve the prescribed amount of sorbitol in an appropriate amount of purified water, heat and control the temperature at 45°C to 50°C, stir for 30 minutes to completely dissolve the sorbitol, and then cool.
[0044] ②Add auxiliary materials: add prescription amount of sodium saccharin, prescription amount of sodium acetate, prescription amount of propylene glycol, prescription amount of glycerin, and prescription amount of propionic acid into the preparation tank.
[0045] ③Adjust the pH value: add an appropriate amount of glacial acetic acid to adjust the pH value to 5.0-5.6 to obtain a matrix solution.
[0046] ④Add the main drug and supplement purified water: add an appropriate amount of purified water, add levocetirizine hydrochloride to it, and stir until the main drug is completely dissolved. Then add pure water to the solution until the volume of the...
Embodiment 9
[0047] Embodiment 9 antibacterial efficacy experiment
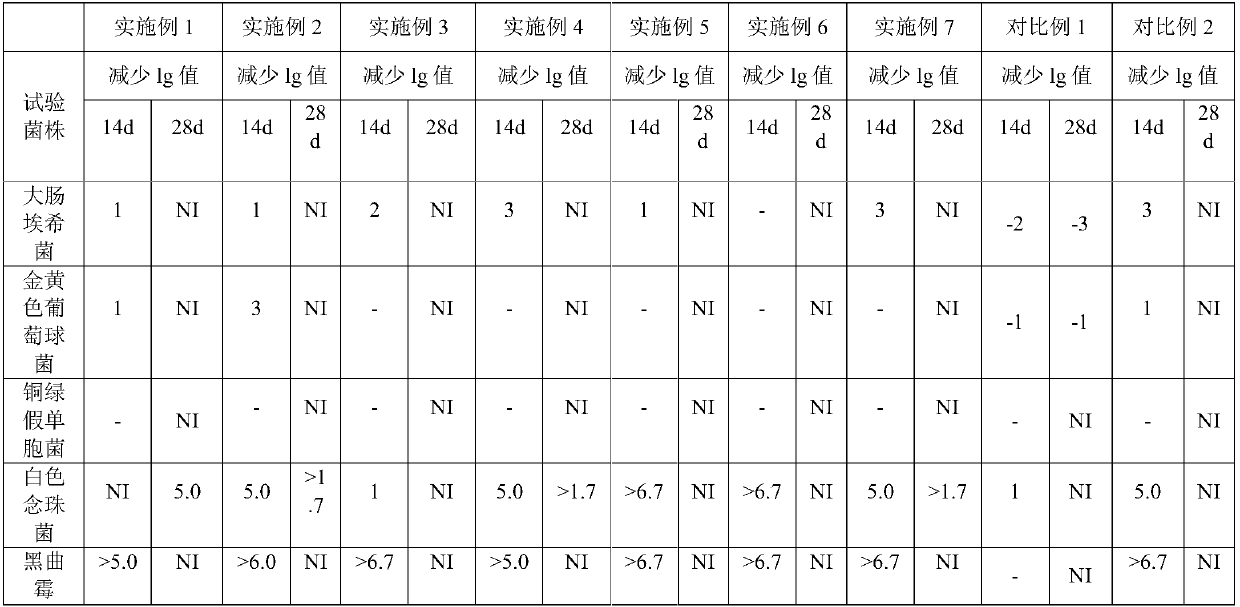
[0048] Examples 1-7 and Comparative Examples 1 and 2 were all prepared according to the above-mentioned process, and their antibacterial efficacy was tested, specifically referring to the antibacterial efficacy inspection method 1121 of the Four General Rules of the Chinese Pharmacopoeia 2015 Edition. The results are shown in Table 2.
[0049] Table 2 Bacteriostatic efficacy test results
[0050]
[0051] The test results show that the levocetirizine hydrochloride oral solution prepared in Examples 1-7 meets the judgment standard of "oral preparation" under the general rule 1121 antibacterial efficacy test method of the Chinese Pharmacopoeia 2015 edition. The effective antibacterial effect can be maintained in the range of 0.05%-0.15% propionic acid volume percentage.
Embodiment 10
[0052] Embodiment 10 taste test experiment
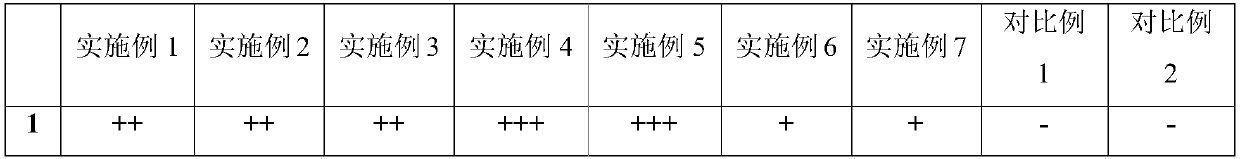
[0053] The mouthfeel test was carried out on 30 healthy subjects in a blind method, and the results are shown in Table 3.
[0054] Table 3 taste test experiment and results
[0055]
[0056]
[0057] Note: +++ means good taste, ++ means good taste, + means average taste, - means poor taste, -- means poor taste.
[0058] In the experimental results, more than half of the 30 subjects rated the mouthfeel of Example 5 as good, and the acceptability of Examples 1, 2, 3, 4, 6, 7 and Comparative Example 1 was better. Mouthfeel evaluations for Example 2 were generally poor and poor. It shows that when the volume percentage of propionic acid exceeds 0.15%, the mouthfeel is sour, and the acceptance among the crowd is low.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com