A kind of preparation method of omeprazole metabolite
A technology of omeprazole and metabolites, which is applied in the field of drug synthesis, can solve the problem that metabolite research has not been reported, and achieves the effect of reasonable route design and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
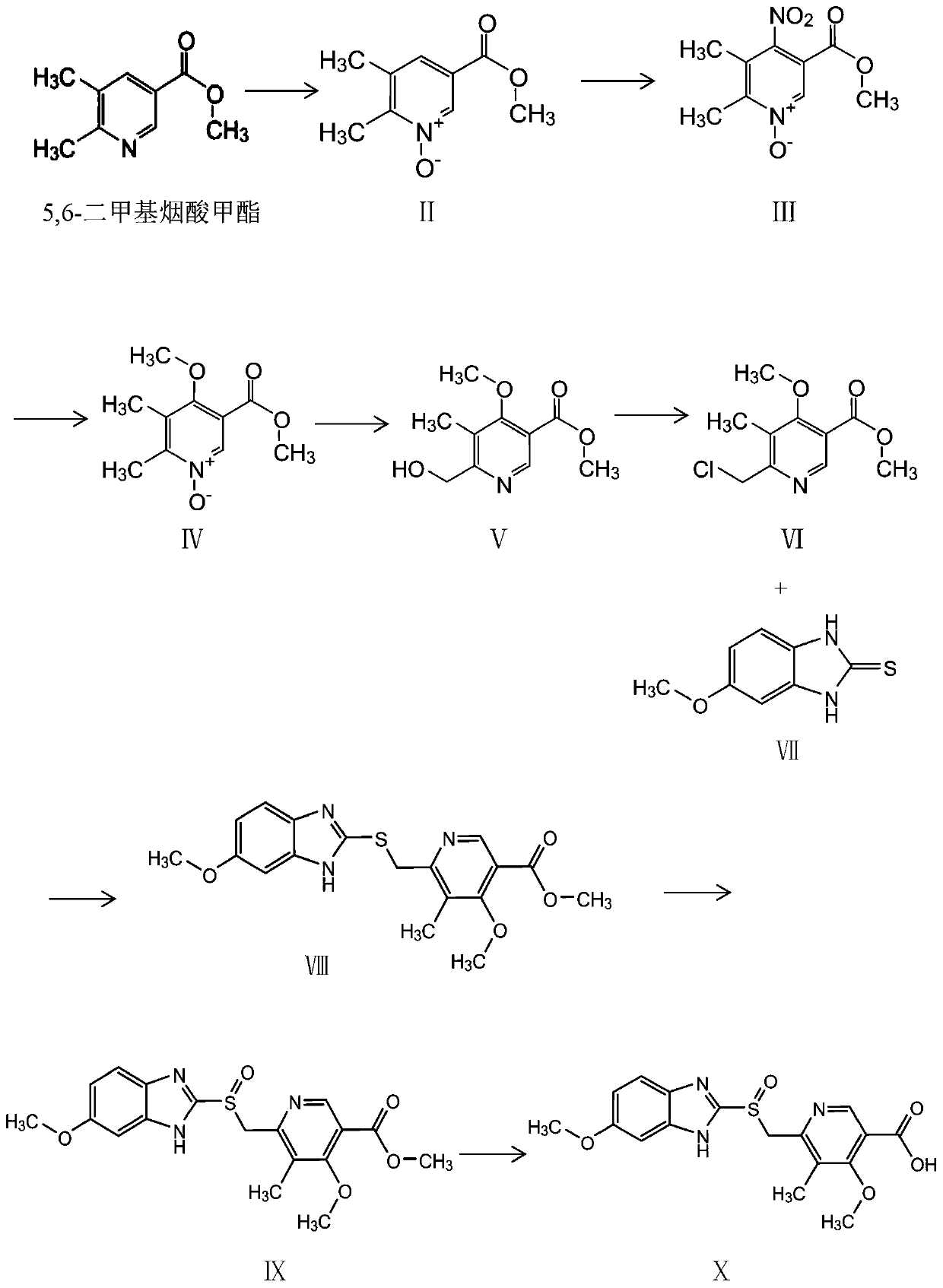
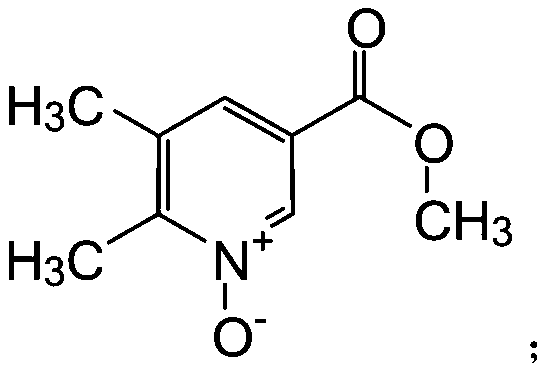
[0027] (1) Take 15g of 5,6-dimethylnicotinic acid methyl ester and dissolve it in 150mL of dichloromethane, add 31g of 75% m-chlorobenzoic acid in batches under ice bath, stir and react at room temperature for 3 hours, Thin-layer chromatography showed that the reaction was complete, and the organic phase was washed with saturated aqueous sodium bicarbonate to obtain 14.64 g of compound II as a yellow solid, with a yield of 89%;
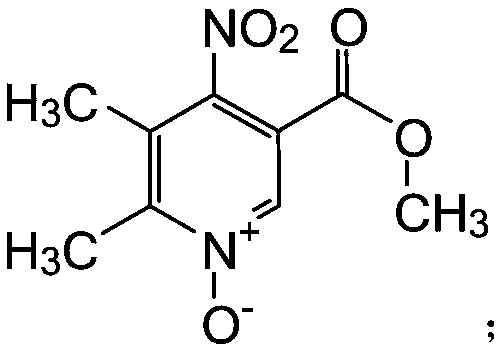
[0028] (2) 6g of Compound II was added in batches to 30ml of 65% concentrated nitric acid in an ice bath, stirred and reacted at 90°C for 3 hours, thin-layer chromatography showed that the reaction was complete, the reaction solution was reduced to 0°C and water was added, and the product was dichloromethane Extraction, and the organic phase was dried with anhydrous sodium sulfate to obtain 5.6 g of compound III, with a yield of 75%;
[0029] (3) 4.5g of compound Compound III was dissolved in 45mL of methanol, 3.2g of sodium methoxide was added under ...
Embodiment 2
[0036] (1) Dissolve 15g of 5,6-dimethylnicotinic acid methyl ester in 150mL of methanol, add 26.8g of 75% m-chlorobenzoic acid in batches under ice bath, stir and react at room temperature for 3 hours, thin Layer chromatography showed that the reaction was complete, and the organic phase was washed with saturated aqueous sodium bicarbonate to obtain 14.64 g of compound II as a yellow solid, with a yield of 85%;
[0037] (2) 6g of Compound II was added in batches to 36ml of 65% concentrated nitric acid in an ice bath, stirred and reacted at 90°C for 3 hours, thin-layer chromatography showed that the reaction was complete, the reaction solution was lowered to 0°C and water was added, and the product was dichloromethane Extraction, the organic phase was dried with anhydrous sodium sulfate to obtain 5.6 g of compound III, with a yield of 74%;
[0038] (3) 4.5g of compound Compound III was dissolved in 45mL of methanol, 3.2g of sodium methoxide was added under ice-cooling, and reac...
Embodiment 3
[0045] (1) Dissolve 15g of 5,6-dimethylnicotinic acid methyl ester in 150mL of methanol, add 26.8g of 30% hydrogen peroxide in batches under ice bath, stir and react at room temperature for 3 hours, thin-layer chromatography shows that the reaction Completely, the organic phase was washed with a saturated aqueous sodium bicarbonate solution to obtain 14.64 g of compound II as a yellow solid, with a yield of 80%;
[0046] (2) 6g of Compound II was added in batches to 24ml of 65% concentrated nitric acid in an ice bath, stirred and reacted at 90°C for 3 hours, thin layer chromatography showed that the reaction was complete, the reaction solution was lowered to 0°C and water was added, and the product was dichloromethane Extraction, the organic phase was dried with anhydrous sodium sulfate to obtain 5.6 g of compound III, with a yield of 74%;
[0047] (3) 4.5g of compound Compound III was dissolved in 45mL of methanol, 3.2g of sodium methoxide was added under ice-cooling, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com