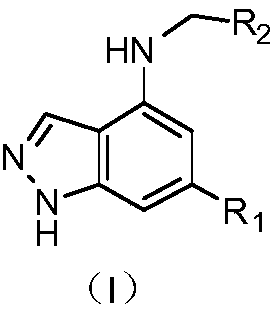
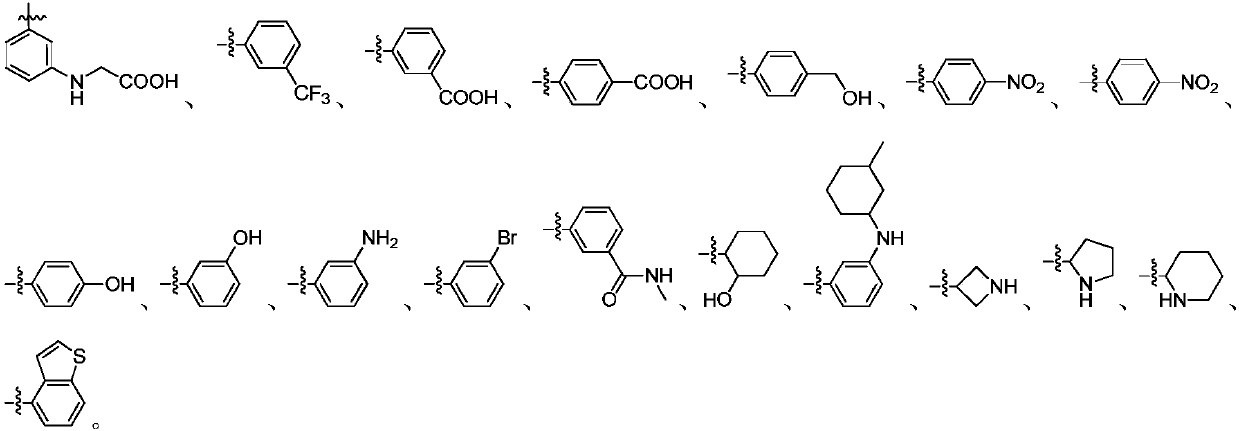
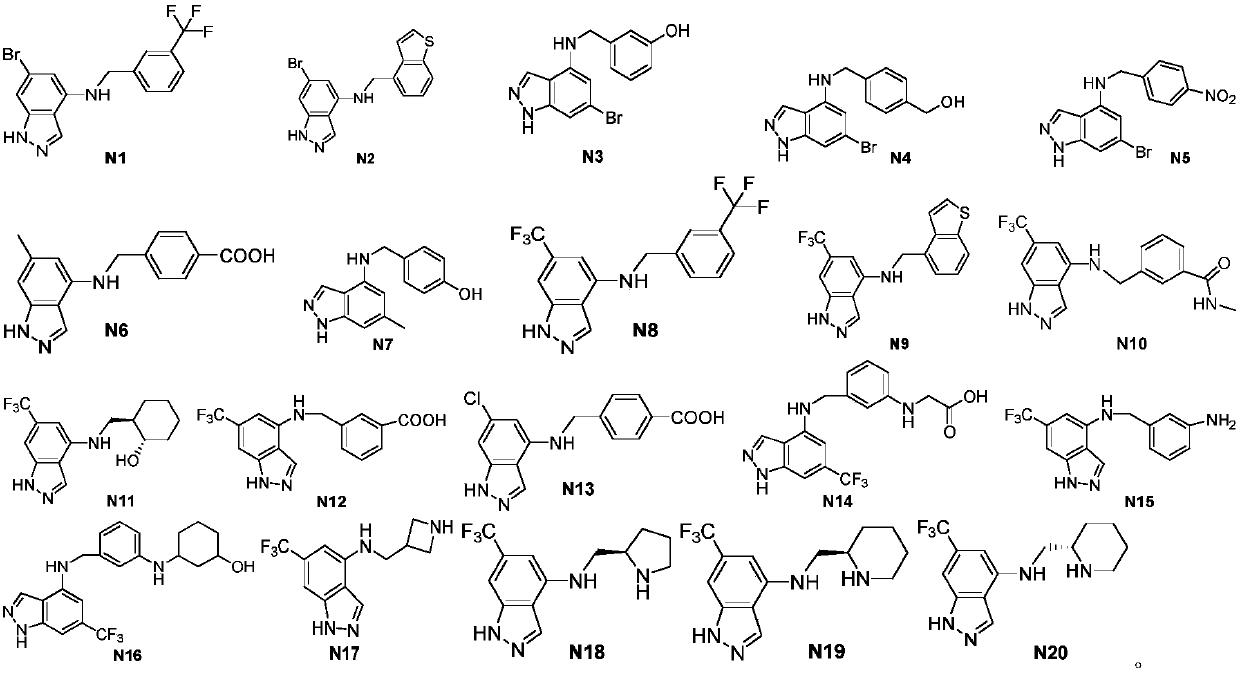
1H-indazole derivatives and application of same as IDO inhibitors
A compound and solvate technology, applied in the field of 1H-indazole derivatives, can solve the problems of reducing tryptophan concentration, inhibiting killing effect, and stagnant synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of intermediate raw materials
[0048] (1) Synthesis of 5a and 5b
[0049]
[0050] Synthesis of 2,5-Dimethyl-1,3-Dinitrobenzene (2a)
[0051] Take a dry 50mL pear-shaped flask, dissolve xylene (1a) (2.00mL, 16.23mmol) with 20mL of concentrated sulfuric acid, slowly add potassium nitrate (4.91g, 48.68mmol) under stirring at room temperature, after the addition, stir at room temperature for 2h, The reaction solution was slowly poured into ice water, filtered, and the filter cake was dried in vacuum, and then purified by column chromatography (PE:EA=80:1) to obtain a pale yellow solid (2a) (1.62 g, yield 51%). Structure Identification: 1 H-NMR (400MHz, CDCl 3 ,ppm):δ13.0(br,1H,NH),7.42(s,2H,Ar-H), 2.45(s,6H,CH 3 ).ESI-MS:197.05[M+H].
[0052] Synthesis of 2-methyl-5-trifluoromethyl-1,3-dinitrobenzene (2b)
[0053] Using 4-trifluoromethyl-toluene (1b) (CAS: 6140-17-6, purchased from Chengdu Ruioke Reagent Company) as raw material, according to the preparation o...
Embodiment 2
[0069] Example 2 Synthesis of compounds N1, N2, N3, N4 and N5 of the present invention
[0070]
[0071] 6-Bromo-N-(3-trifluoromethylbenzyl)-1H-indazol-4-amine (N1).
[0072] Amine 5c (0.28mmol) and benzaldehyde 6 (0.24mmol) were dissolved in dichloromethane (DCM, 3mL), DHP (83.5mg, 0.33mmol) and appropriate amount (840.2 mg), trifluoroacetic acid (TFA, 17.6 μL, 0.24 mmol) was added dropwise, and refluxed at 40° C. for 12 hours, the reaction solution was filtered, spin-dried, and passed through a column (PE:EA=10:1) to obtain compound N1. Yield 53.9%; red powdery solid; 1 H-NMR(400MHz,d 6 -CDCl 3 , ppm): δ8.00 (s, 1H, indazole-H3), 7.69 (s, 1H, Ar-H), 7.63-7.50 (m, 3H, Ar-H), 7.06 (s, 1H, Ar-H ),6.34(s,1H,Ar-H),4.56(d,2H,J=4.4Hz,benzyl-CH 2 ). 13 C-NMR(100MHz,d 6 -CDCl 3 ,ppm):δ141.9,141.7,139.2,131.7,131.4,130.8,129.4,124.6,124.3,122.9,112.5,103.5,102.2,47.6.ESI-MS:370.01[M+H].
[0073] Select the corresponding aldehyde raw materials and prepare the compounds N2, N3, N4, N5 accord...
Embodiment 3
[0082] Example 3 Synthesis of compounds N6 and N7 of the present invention
[0083]
[0084] 4-((6-Methyl-1H-indazole-4-amino)methyl)benzoic acid (N6).
[0085] Compound N6 was synthesized according to the similar method in Example 2, except that compound 5a was substituted for compound 5c to obtain compound N6. Yield 42.0%; light yellow powdery solid; 1 H-NMR(400MHz,d 6 -DMSO, ppm): δ12.72 (s, 2H, COOH and indazole-NH), 8.12 (s, 2H, indazole-H3 and NH), 7.91 (d, 2H, J = 8.1 Hz, Ar-H), 7.50 (d,2H,J=8.1Hz,Ar-H),6.47(s,1H,Ar-H),5.77(s,1H,Ar-H),4.50(s,2H,benzyl-CH 2 ),2.21(s,3H,CH 3 ),ESI-MS:282.12[M+H].
[0086] 4-((6-Methyl-1H-indazole-4-amino)methyl)benzyl alcohol (N7).
[0087]
[0088] Select the corresponding aldehyde raw materials, and prepare compound N7 according to a similar method with a yield of 53.7%; a light yellow powdery solid; 1 H-NMR(400MHz,d 6 -DMSO, ppm): δ9.23(s,2H,indazole-NH and OH), 8.12(s,1H,indazole-H), 7.19(d,2H,J=8.4Hz,Ar-H), 6.72( d, 2H, J = 8.4 Hz, Ar-H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com