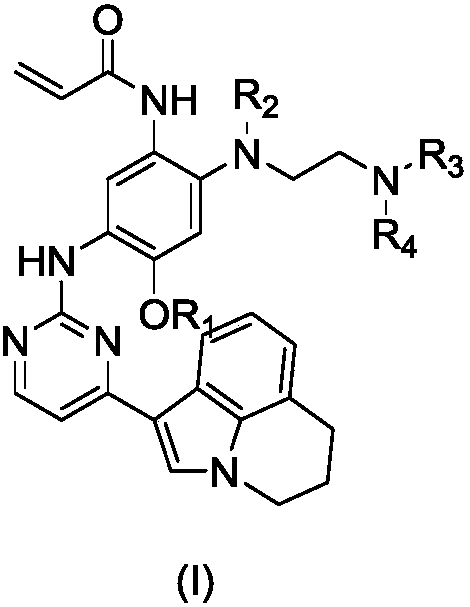
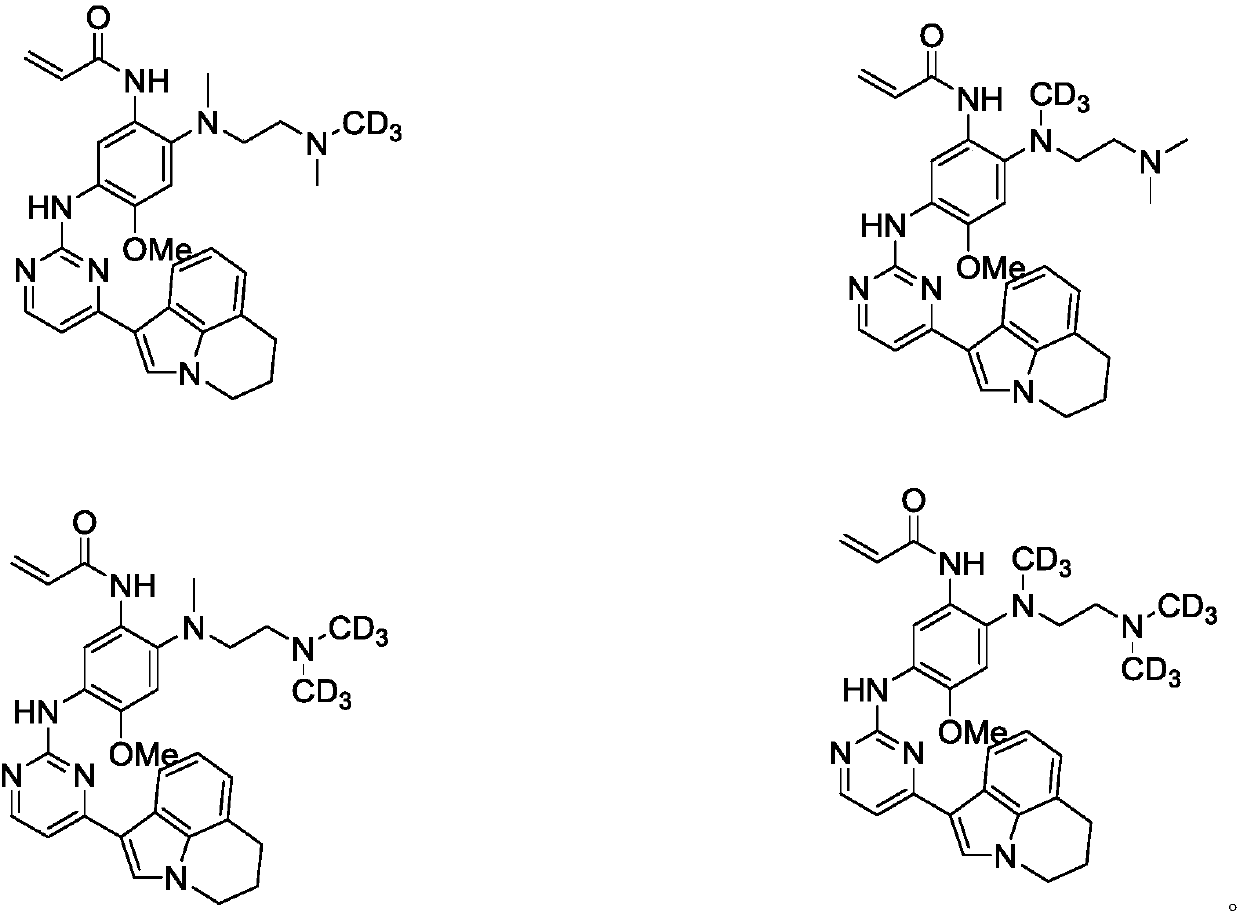
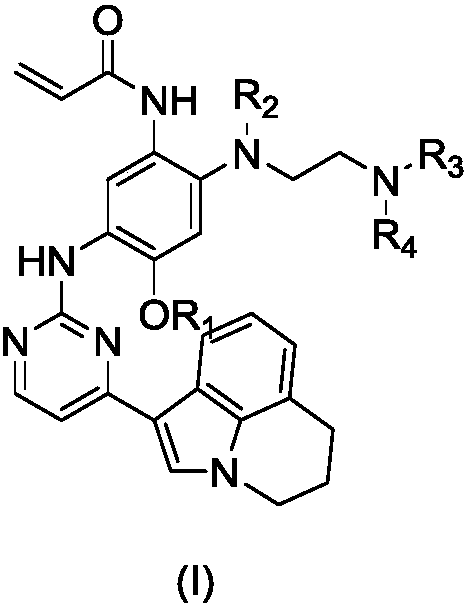
Deuterated 3-(4,5-substituted amino pyrimidine)phenyl derivatives, and applications thereof
A use and drug technology, applied in the field of anti-tumor drugs, can solve the problems of wild-type cytotoxic side effects, unable to solve the clinical needs of drug resistance, and low clinical tolerance of drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] The synthetic route is as follows:
[0041]
[0042] Compound 1
[0043]
[0044] Take a 250mL single-necked flask, add N-methylethanolamine (10g, 133.1mmol), TEA (26.9g, 266.3mmol), acetonitrile (100mL) respectively, and then slowly add benzyl chloride (23.9g, 139.8mmol) at 0°C Slowly added dropwise to the reaction solution, and continued to stir at room temperature for 1 h. TLC monitored that no raw material remained, and the solvent was distilled off under reduced pressure, and purified by column chromatography to obtain 21 g of a colorless liquid, namely compound 1, with a yield of 95.5%.
[0045] Compound 2
[0046]
[0047] Take a 250mL eggplant-shaped bottle, add compound 1 (21g, 127.1mmol), TEA (25.7g, 254.2mmol) and DCM (100ml), and then add MsCl (14.6g, 127.1mmol) dropwise at 0°C. The reaction solution was stirred at room temperature for 3 h, no raw materials were detected by TLC, the solvent was evaporated under reduced pressure, and pu...
Embodiment 2
[0078]
[0079] The synthetic route is as follows:
[0080]
[0081] Compound 12
[0082]
[0083] Take 120mL sealed tube and add compound 8 (500mg, 1.19mmol), N-methylethanolamine (107.26mg, 1.42mmol), DIPEA (307.59mg, 2.38mmol), DMA (5mL). Then seal the tube and react at 140°C for 6 hours. TLC monitors that there is no raw material remaining. Cool the reaction solution to room temperature, add 20mL of water, precipitate a solid, filter, then add the filter cake to 2mL of methanol for beating and washing, filter and dry to obtain 480mg of reddish-brown solid Namely compound 12, the yield is 85%.
[0084] Compound 13
[0085]
[0086] Take a 20 mL eggplant-shaped bottle and add compound 12 (450 mg, 1.05 mmol), TEA (159.39 mg, 1.57 mmol) and DCM (5 ml). Slowly add MsCl (120.28mg, 1.05mmol) dropwise at 0°C. After the dropwise addition, stir at this temperature. After 1h, TLC monitors that there is no raw material remaining. The solvent is distilled off under reduc...
Embodiment 3
[0098]
[0099] The synthetic route is as follows:
[0100]
[0101] Compound 17
[0102]
[0103] Take a 2L three-necked bottle and add CD respectively 3 OH (15 g, 415.8 mmol), THF (600 mL). Then n-BuLi (174.6 mL, 436.6 mmol) was slowly added dropwise at -40°C. After stirring for 1 h, a tetrahydrofuran solution of TsCl (79.3 g, 415.8 mmol) was added dropwise. After the addition was completed, stirring was continued for 3 h, and no raw material remained as monitored by TLC. Add 600mLH 2 O quenching, EA extraction (200mL*3), brine washing, anhydrous Na 2 SO 4 After drying, the organic phases were combined, and the solvent was distilled off under reduced pressure to obtain a crude compound, which was purified by column chromatography to obtain 73 g of a white solid, compound 17, with a yield of 92.7%.
[0104] Compound 18
[0105]
[0106] Take a 250mL eggplant-shaped bottle and add compound 17 (8g, 42.3mmol), N-benzylethanolamine (5.3g, 35.2mmol), triethylamine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com