Selective solvent free phosphorylation
A technology selected from compounds, applied in the direction of non-central analgesics, biocides, anti-inflammatory agents, etc., can solve problems such as hindering implementation and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
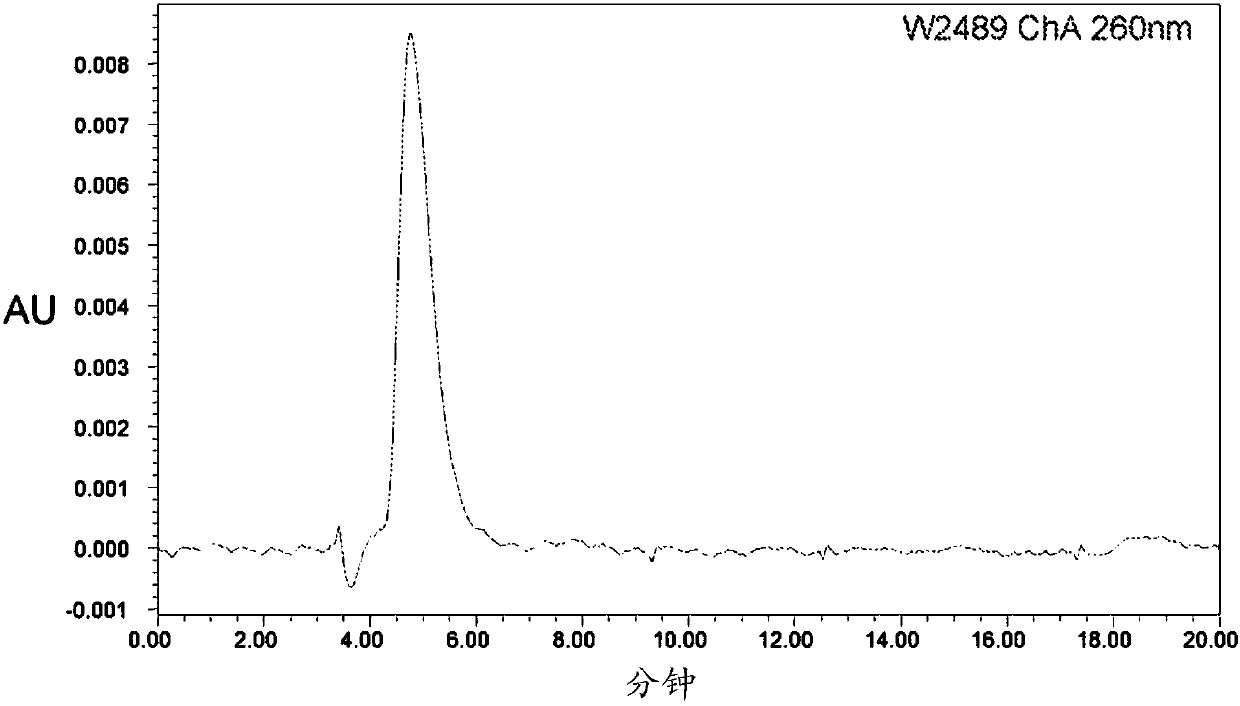
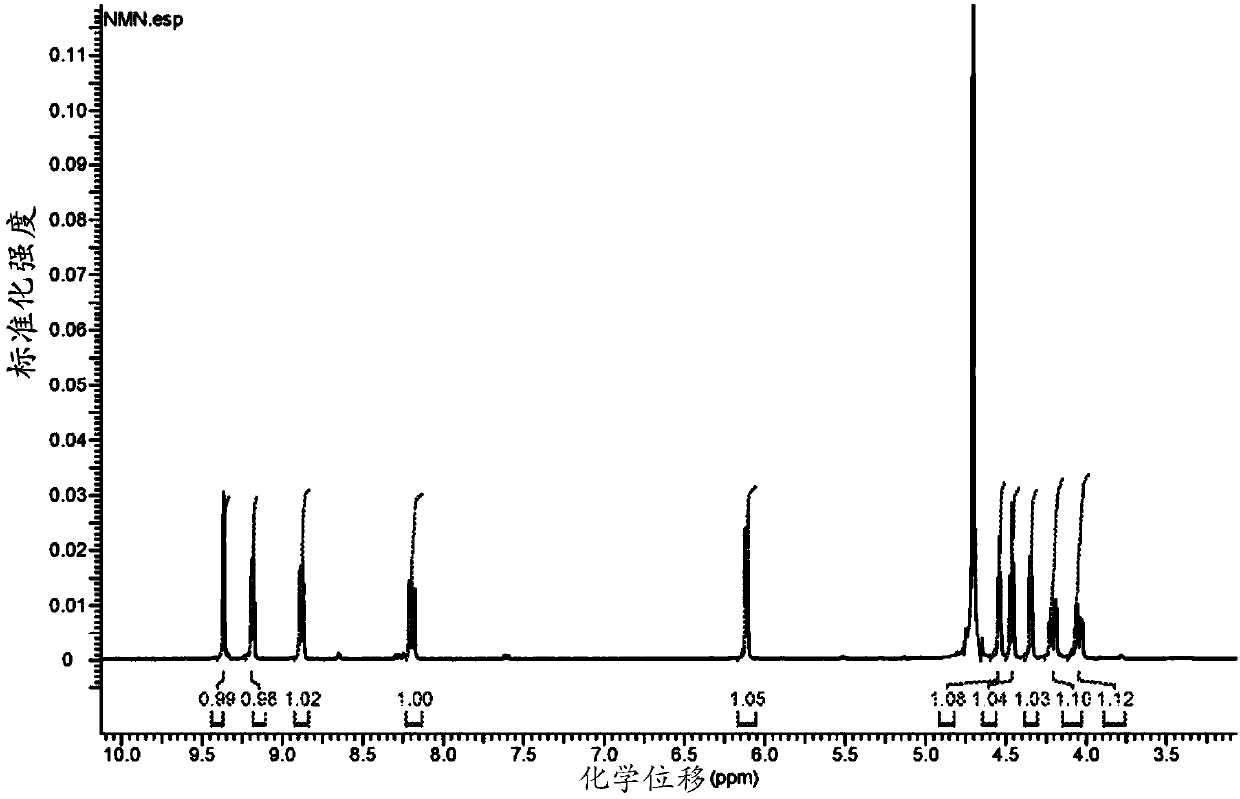
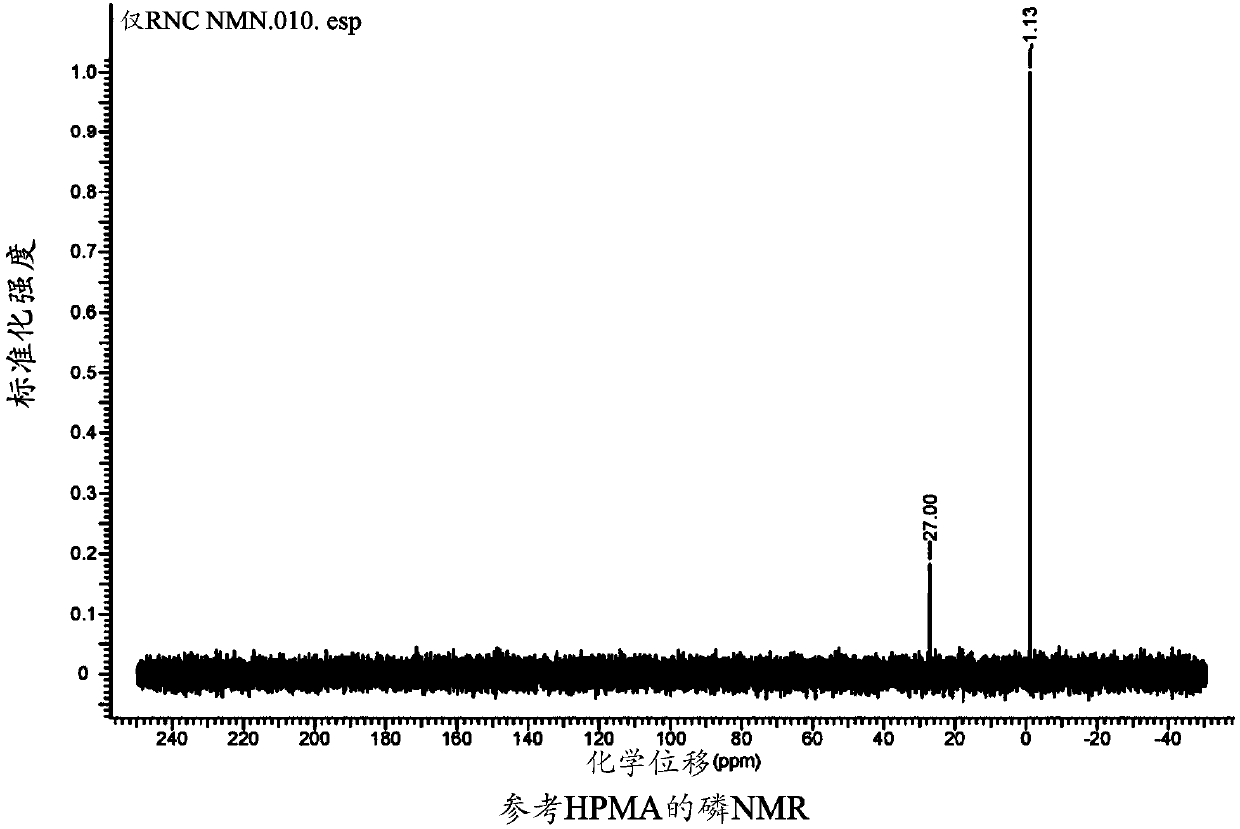
[0241] The synthetic preparation of nicotinamide mononucleotide (NMN): the compound of formula (I): R 1 = hydrogen, n = 0, Z 2 =NH,R 2 -R 5 = Hydrogen, Y 1 = sodium, Y 2 = Inner salt with pyridinium.
[0242]
[0243] Nicotinamide Mononucleotide (NMN)
[0244] To a dry 35 mL PTFE milling vessel containing one PTFE sphere (0.8 cm diameter) was added nicotinamide riboside chloride (2000 mg, 6.88 mmol, 1.0 equiv) and POCl 3 (2.57 mL, 27.52 mmol, 4.0 equiv). The reaction was then milled at 30 Hz for 60 minutes or until the reaction had reached approximately 95% conversion via c-18 HPLC analysis. The gelled, gummed sphere was removed and placed in a wide neck flask, and the residue was dissolved in a minimal volume of distilled water on ice. The solution was adjusted to pH 6.0 by dropwise addition of 2M NaOH solution. The aqueous solution was then reduced to a small volume under high vacuum, and the pH was then adjusted to pH 3.0 using dilute nitric acid. Aceto...
example 2
[0258] Synthetic preparation of thiamine monophosphate.
[0259]
[0260] Thiamine monophosphate
[0261] To a dry 35 mL PTFE milling vessel containing one PTFE sphere (0.8 cm diameter) was added thiamine hydrochloride (2000 mg, 6.63 mmol, 1.0 equiv) and POCl 3 (2.43 mL, 26.51 mmol, 4.0 equiv). The reactants were then milled at 30 Hz for 60 minutes, or until the 1 H-NMR analysis The reaction has reached near completion. The white pasty residue was then dissolved in a minimum volume of distilled water on an ice bath, and then concentrated to give a fluffy white powder (92% conversion).
[0262] 1 H NMR (400MHz,D 2 O)δppm 9.51(s,1H,Ar),7.79(s,1H,Ar),5.38(s,2H),4.46(q,J=5.4Hz,2H),3.15(t,J=4.9Hz,2H ), 2.43(s,3H), 2.36(s,3H). 13 C NMR (125MHz,D 2 O) δ ppm 163.2, 163.0, 155.0, 154.9, 144.3, 143.3, 135.5, 106.2, 64.8, 49.9, 27.5 (d, J = 10.3 Hz), 20.9, 11.1. 31 P NMR (162MHz,D 2 O) δppm-0.62.
[0263] pass 1 The chemical shifts observed on H NMR at 2.99, 3.69, 7.83 a...
example 3
[0265] Synthesis and Preparation of Pyridoxine Monophosphate
[0266]
[0267] pyridoxine monophosphate
[0268] Pyridoxine (500mg, 2.99mmol, 1.0 equiv) and POCl 3 (1.12ml, 11.96mmol, 4.0eq) was added to a 50mL ceramic mortar and the mixture was then manually ground using a ceramic pestle for a total of 30 minutes. 1 H NMR analysis showed 20% conversion to the desired product. 1 H NMR (400MHz,D 2 O) δppm 8.01 (1H, s, aldehyde), 6.84 (1H, s, Ar), 5.24 (1H, m, CH 2 O),5.10(1H,m,CH 2 O),2.49(3H,s,CH 3 ). 31 P NMR (162MHz,D 2 O) δppm-1.03.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com