Reactor and method for continuously producing nitromethane
A nitromethane and reactor technology, applied in the field of reactors for continuous production of nitromethane, can solve the problems of low product purity, low yield, and easy side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
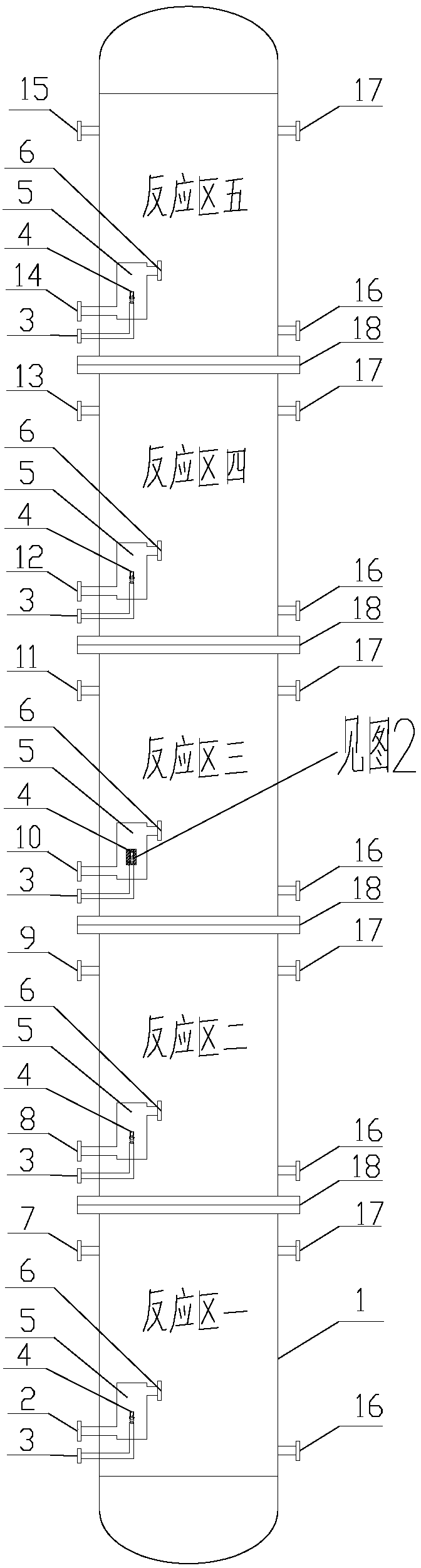
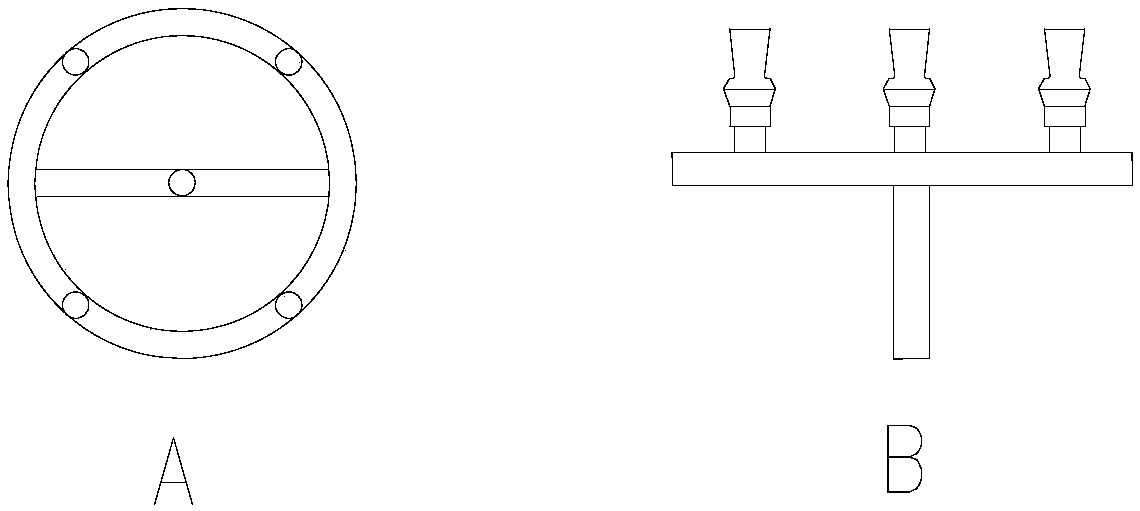
[0030] Sodium nitrite aqueous solution is fed continuously through the inlet 2, and dimethyl sulfate is injected into the mixing chamber 5 through the inlet 3 of the reaction zone 1 through the mixed flow nozzle 4 and mixed with the sodium nitrite solution (feed section n=1) , the molar ratio of dimethyl sulfate and sodium nitrite is 2:1. The two materials are mixed quickly and evenly in the mixing chamber under the action of the mixed-flow nozzle, and then enter the reaction tube connected to the 6-phase for efficient reaction. During the reaction, the circulation of the cooling water is realized through the heat transfer medium inlet 16 and the heat transfer medium outlet 17, and the reaction heat generated by the reaction is removed, so that the reaction solution is continuously incubated at 60°C. After the reaction, the reaction product is continuously transported to the subsequent separation workshop through the outlet 7 for refining and purification to obtain the nitrome...
Embodiment 2
[0032] Sodium nitrite aqueous solution is passed into continuously through inlet 2, and the dimethyl sulfate of 50% quality passes through the inlet 3 of reaction zone one and sprays into mixing chamber 5 and mixes with sodium nitrite solution through mixing nozzle 4 (feed section n=2), the molar ratio of dimethyl sulfate total amount and sodium nitrite is 2:1. The two materials are mixed quickly and evenly in the mixing chamber under the action of the mixed-flow nozzle, and then enter the reaction tube connected to the 6-phase for efficient reaction. Then reaction solution leaves reaction zone one by outlet 7, and enters reaction zone two by inlet 8, carries out rapid mixing with the dimethyl sulfate (50% quality) that comes from inlet 3 and injects through mixing nozzle 4 in mixing chamber 5, then Enter the reaction tube connected with 6 phases for high-efficiency reaction. During the reaction, the heat transfer medium inlet 16 and the heat transfer medium outlet 17 of the ...
Embodiment 3
[0034]Sodium nitrite aqueous solution is passed into continuously by inlet 2, and the dimethyl sulfate of 30% quality passes through the inlet 3 of reaction zone one and sprays into mixing chamber 5 and mixes with sodium nitrite solution through mixed flow nozzle 4 (feed section n=3), the molar ratio of dimethyl sulfate total amount and sodium nitrite is 2:1. The two materials are mixed quickly and evenly in the mixing chamber under the action of the mixed-flow nozzle, and then enter the reaction tube connected to the 6-phase for efficient reaction. Then reaction solution leaves reaction zone one by outlet 7, and enters reaction zone two by inlet 8, carries out rapid mixing with the dimethyl sulfate (30% quality) that comes from inlet 3 and sprays into through mixing nozzle 4 in mixing chamber 5, then Enter the reaction tube connected with 6 phases for high-efficiency reaction. Then reaction solution leaves reaction zone two through outlet 9, and enters reaction zone three by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com