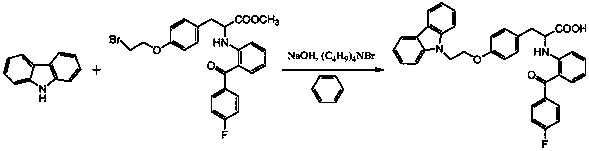
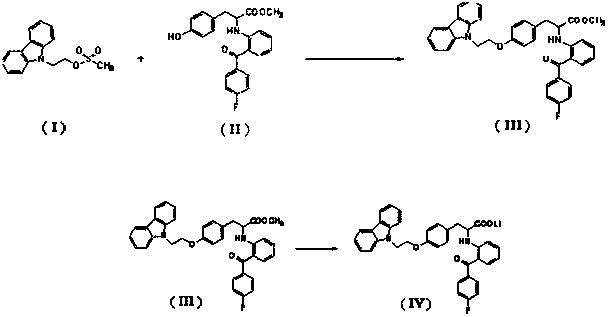
Preparation method of phenylalanine compound
A compound, the technology of propionic acid, which is applied in the field of preparation of phenylalanine compounds, can solve the problems of high impurity content, many side reactions, and inability to carry out large-scale industrial production, and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
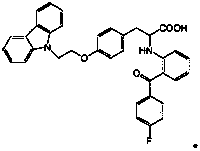
[0037] Example 1: 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionic acid preparation
[0038]
[0039] Add 400mL toluene, 39.34g (100mmol) 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propionic acid methyl ester, 43.40g (150mmol) 9-carbazole ethanol mesylate and 39.40g (120mmol) cesium carbonate were reacted at 90°C for 3 hours, filtered, and the filtrate was concentrated in vacuo to remove the solvent toluene to obtain 2-[(2-(4-fluorobenzene Formyl)phenyl)amino]-3-(4-hydroxyphenyl)propanoic acid methyl ester crude product, purity (HPLC) 69.8%, LC-MS (m / z) 587 (M+1). The obtained crude product was directly used in the next reaction without further purification.
[0040] Add the crude product of methyl 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propionate and 400 mL of tetrahydrofuran into the reaction flask, and stir to dissolve at room temperature. 16.78g (400mmol) LiOH.H2O was dissolved in 200mL of water, added...
Embodiment 2
[0041] Example 2: 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionic acid preparation
[0042]
[0043] Add 40mL toluene, 3.93g (10mmol) 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propionic acid methyl ester, 4.34g (15mmol) 9-carbazole ethanol mesylate and 3.95g (12mmol) cesium carbonate were reacted at 80°C for 2 hours, filtered, and the filtrate was concentrated in vacuo to remove the solvent toluene to obtain 2-[(2-(4-fluorobenzene Formyl)phenyl)amino]-3-(4-hydroxyphenyl)propanoic acid methyl ester crude product, LC-MS (m / z) 587 (M+1). The obtained crude product was directly used in the next reaction without further purification.
[0044] Add the crude 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propanoic acid methyl ester and 40mL tetrahydrofuran into the reaction flask, and stir at room temperature to dissolve. 1.68g (40mmol) LiOH.H2O was dissolved in 20mL of water, added to the above solution, stirred and rea...
Embodiment 3
[0045] Example 3: 2-(2-(4-fluorobenzoyl)phenylamino)-3-(4-(2-(9H-carbazol-9-yl)ethoxy)phenyl)propionic acid preparation
[0046]
[0047] Add 40mL toluene, 3.93g (10mmol) 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propionic acid methyl ester, 4.34g (15mmol) 9-carbazole ethanol mesylate and 3.95g (12mmol) cesium carbonate were reacted at 120°C for 2 hours, filtered, and the filtrate was concentrated in vacuo to remove the solvent toluene to obtain 2-[(2-(4-fluorobenzene Formyl)phenyl)amino]-3-(4-hydroxyphenyl)propanoic acid methyl ester crude product, LC-MS (m / z) 587 (M+1). The obtained crude product was directly used in the next reaction without further purification.
[0048] Add the crude 2-[(2-(4-fluorobenzoyl)phenyl)amino]-3-(4-hydroxyphenyl)propanoic acid methyl ester and 40mL tetrahydrofuran into the reaction flask, and stir at room temperature to dissolve. 1.68g (40mmol) LiOH.H2O was dissolved in 20mL of water, added to the above solution, stirred and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com