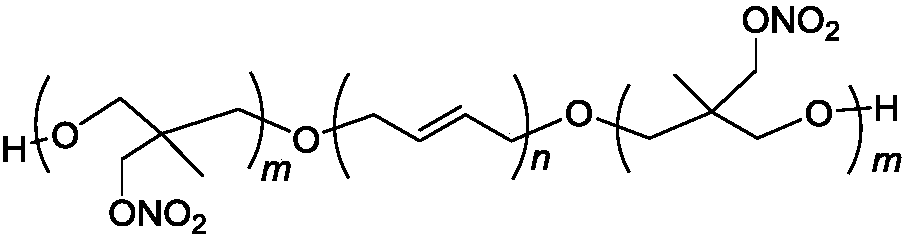
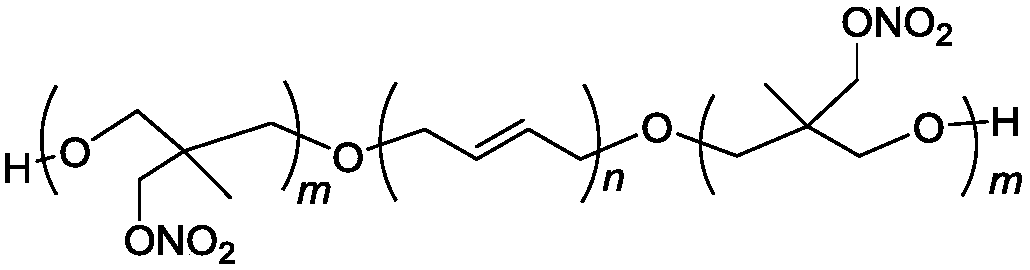
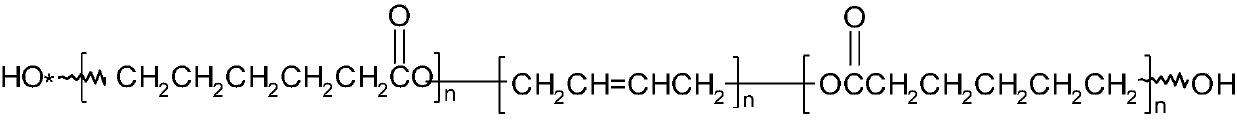Triblock energetic nitrate adhesive and synthesis method thereof
A synthesis method and nitrate technology, applied in the field of solid propellants, can solve problems such as affecting the energy of propellant formula, and achieve the effect of increasing miscibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 150mL four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 10g (1.7mmol) HTPB and 20mL dichloromethane were added successively, and 0.5mL boron trifluoride·ether was added after stirring to dissolve. The complex is reacted for 30 minutes at room temperature. Add 5.72g (40mmol) 3-nitrate methyl-3-methyloxetane (NIMMO) dropwise, the dropping time is 4h, the reaction temperature is controlled at 5~10℃, after dropping, polymerize at room temperature for 12h, use The saturated sodium carbonate aqueous solution was used to terminate the reaction, and the reaction solution was poured into 50 mL of water in a stirring state, and the lower organic phase was separated and washed three times with 50 mL of water. Separate the oil phase, add anhydrous magnesium sulfate to dry, stand for filtration, and remove dichloromethane and other volatile substances under reduced pressure to obtain 15.05 g of viscous liquid.
[...
Embodiment 2
[0034] Into a 150mL four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 10g (1.7mmol) HTPB and 20mL dichloromethane were sequentially added, and after stirring, 0.5mL boron trifluoride·ether was added. The complex is reacted for 30min at room temperature. 11.44g (80mmol) 3-nitrate methyl-3-methyloxetane (NMMO) was added dropwise, the dropping time was 8h, the reaction temperature was controlled at 5~10℃, and the polymerization was carried out at room temperature for 24h after dropping. The reaction was terminated by saturated sodium carbonate aqueous solution, the reaction solution was poured into 50 mL of water in a stirring state, and the lower organic phase was separated and washed three times with 50 mL of water. Separate the oil phase, add anhydrous magnesium sulfate to dry, stand for filtration, and remove dichloromethane and other volatile substances under reduced pressure to obtain 20.68 g of viscous liqui...
Embodiment 3
[0037] Into a 150mL four-necked round bottom flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, 10g (1.7mmol) HTPB and 20ml dichloromethane were sequentially added, and after stirring, 0.5mL boron trifluoride·ether was added. The complex is reacted for 30 minutes at room temperature. 22.87g (40mmol) 3-nitrate methyl-3-methyloxetane (NIMMO) was added dropwise, the dropping time was 8h, the reaction temperature was controlled at 5~10℃, and the polymerization was carried out at room temperature for 24h after dropping. The saturated sodium carbonate aqueous solution was used to terminate the reaction, and the reaction solution was poured into 50 mL of water in a stirring state, and the lower organic phase was separated and washed three times with 50 mL of water. Separate the oil phase, add anhydrous magnesium sulfate to dry, stand for filtration, and remove dichloromethane and other volatile substances under reduced pressure to obtain 32.66 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com