Double-open-loop derivatives and preparation methods of cyperapuinone and analogues and application in CIK cell culture
A double ring-opening and derivative technology, which is applied in the direction of biochemical equipment and methods, cell culture active agents, and the preparation of organic compounds, can solve the problems that have not been disclosed for the proliferation of sedgequinone and the promotion of stem cell differentiation, and there is no single furan ring. , reports on ring-opening derivatives of bisfuran rings, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
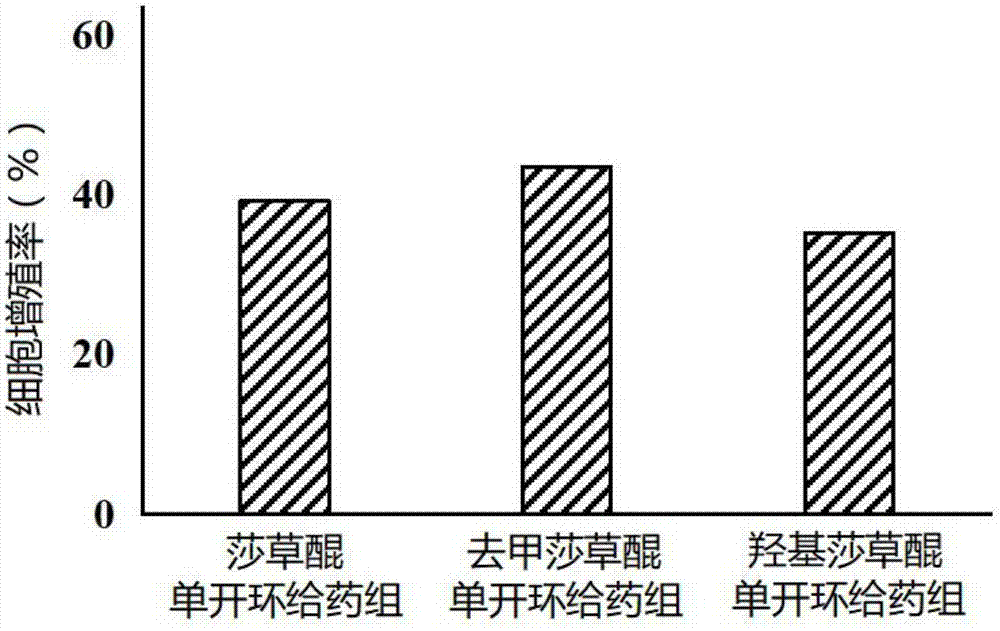
[0046] Example 1: Preparation of single ring-opened derivatives of sedgequinone and its analogues
[0047] First, add syringoquinone, norsyringoquinone, and hydroxysyringoquinone respectively to acidic aqueous solution (deionized water is adjusted to pH 6.3 with hydrochloric acid, of course other volatile acids such as nitric acid or formic acid can also be used instead), 4 mg of starting material was added to 1 mL of acidic aqueous solution. Then move the solution into a reaction kettle, seal it and carry out a hydrothermal reaction at a temperature of 180° C. for a reaction time of 10 hours, and naturally drop to normal temperature after the reaction is completed. Finally, heat and concentrate, freeze-dry when the pH of the solution is close to neutral to obtain the single-ring-opening derivatives of syringoquinone, the single-ring-opening derivatives of norsyringoquinone, and the single-ring-opening derivatives of hydroxysyringoquinone, all with a purity of 95 %above. See...
Embodiment 2
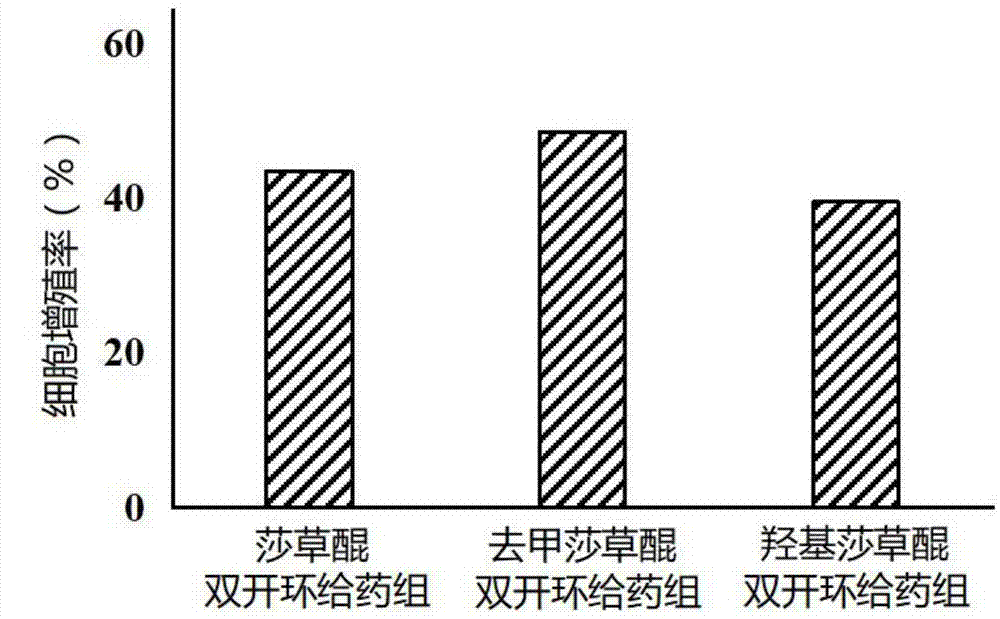
[0048] Example 2: Preparation of double ring-opened derivatives of sedgequinone and its analogues
[0049] First, add syringoquinone, norsyringoquinone, and hydroxysyringoquinone respectively to acidic aqueous solution (deionized water is adjusted to pH 5.4 with hydrochloric acid, of course other volatile acids such as nitric acid or formic acid can also be used instead), 4 mg of starting material was added to 1 mL of acidic aqueous solution. Then move the solution into a reaction kettle, seal it and carry out a hydrothermal reaction at a temperature of 180° C. for a reaction time of 10 hours, and naturally drop to normal temperature after the reaction is completed. Finally, heat and concentrate, and freeze-dry when the pH of the solution is near neutral to obtain syringoquinone double-ring-opening derivatives, norsyringoquinone double-ring-opening derivatives, and hydroxysyringoquinone double-ring-opening derivatives, all of which have a purity of more than 95%. See Table 1-...
Embodiment 3
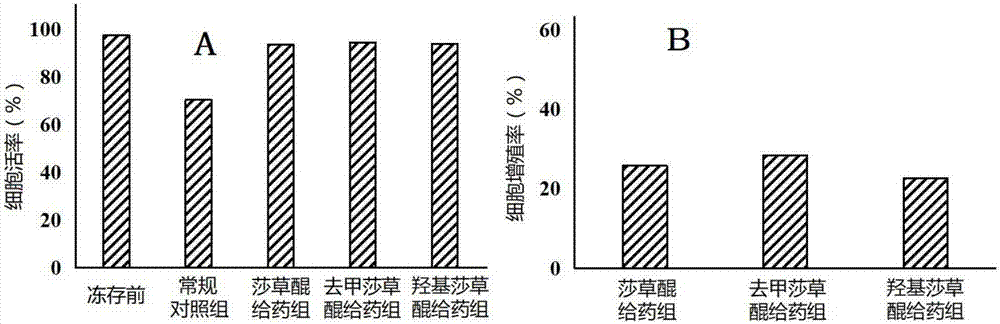
[0056] Example 3: Cycloquinone and its analogues improve CIK cell cryopreservation recovery rate
[0057] 1. Experimental materials
[0058] AIM-VCTS TM The medium was purchased from Gibco, recombinant human interleukin 2 (rh IL-2) was purchased from Shuanglu Pharmaceutical; lymphocyte separation fluid was purchased from Nycomed Pharma (Nycomed PharmaAS, Oslo, Norway), and APC-labeled anti-CD56 ( CD56-APC), PE-labeled CD4 (CD4-PE), PerCP-labeled CD3 (CD3-PerCP), APC-H7-labeled CD8 (CD8-APC-H7) monoclonal antibodies were all purchased from BD Company.
[0059] 2. CIK cell isolation, culture expansion, flow detection
[0060] The peripheral blood of healthy volunteers was drawn into a centrifuge tube containing sodium heparin, and the serum and whole blood cells were obtained by centrifugation, and the whole blood cells were subjected to density gradient centrifugation to obtain mononuclear cells (PBMCs). Harvested PBMCs were resuspended in AIM-VCTS containing 10% inactivated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com