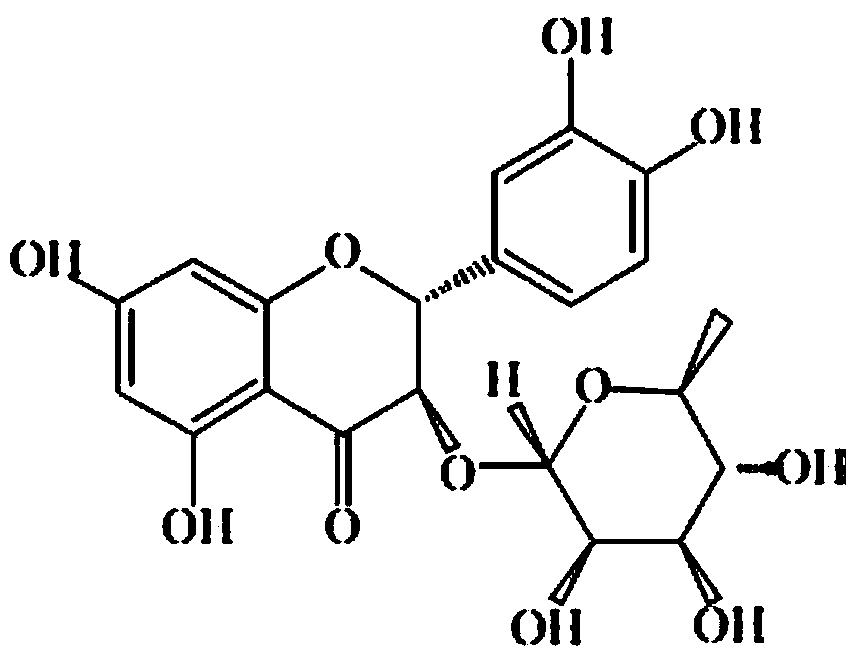
Application of astilbin to preparation of medicine for treating diabetic gangrene
A technology of diabetic gangrene and astilbin, which is applied in the application field of astilbin in the preparation of drugs for treating diabetic gangrene, can solve the problems of large side effects and low compliance of diabetic gangrene, and achieve improved compliance, low side effects, slow down The effect of development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
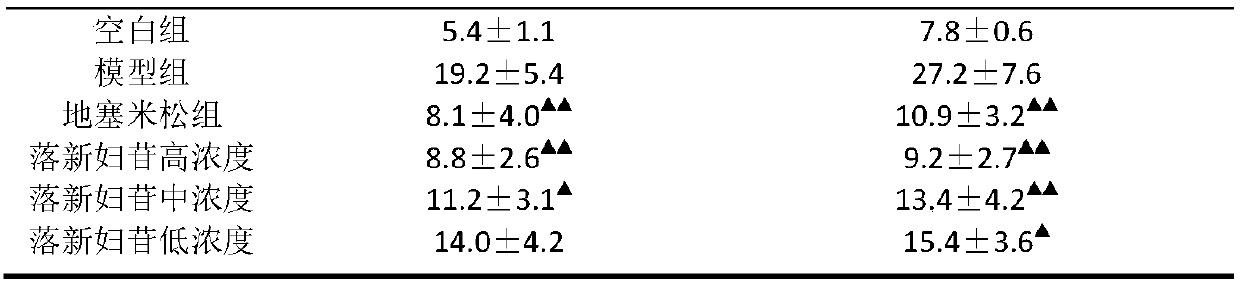
[0018] Astilbin inhibits the secretion of inflammatory factors in macrophages in vitro
[0019] The mouse peritoneal macrophage cell line Raw 264.7 was cultured in a cell culture incubator with DMEM medium containing 10% fetal bovine serum. After the cells grow well, digest the cells, and dilute 100 μL of the cell suspension according to 1×10 6 / L concentration was inoculated into 96-well plates, and then divided into blank control group, model group, and dexamethasone group (10 -7 mol / L) and astilbin high concentration group (10 -5 mol / L), medium concentration group (10 -6 mol / L), low concentration group (10 -7 mol / L), and 5 wells were made for each concentration group. Except for the blank group which was added with 100 μL of PBS, the rest of the groups were stimulated with 5 mg / L lipopolysaccharide LPS. After incubation for 48 hours, the cell supernatant was collected and centrifuged to detect the contents of TNF-α and IL-6 in the supernatant according to the instructi...
Embodiment 2
[0026] Experiment of astilbin reducing wound area in diabetic gangrene mice
[0027] 50 mice were randomly divided into 5 groups, 10 in each group, respectively high-dose metformin group (200mg / kg), low-dose metformin group (50mg / kg), high-dose astilbinin group (200mg / kg), Astilbin low dose group (50mg / kg) and model group. The mice in each group were intraperitoneally injected with streptozotocin (STZ) 35 mg / kg every day for 4 consecutive days. After 4 weeks, a full-thickness wound (2mm×4mm) was prepared on the skin of the back of the lower limb and near the leg of the mouse after anesthesia to establish a mouse model of diabetic gangrene. After the operation, the rats were given intragastric administration once a day, and the model group was given the same volume of drinking water by intragastric administration. At the same time, the wound healing of the mice in each group was observed daily, and the wounds were photographed on the 15th day, and the wound area was measured ...
Embodiment 3
[0033] Wang Moumou, male, 51 years old, has a history of diabetes for 10 years. When the patient came to the doctor, the skin color of his feet was dark, the dorsum of his feet was red and swollen, the little toes of his feet were purple and black with ulceration, and the pulse of the dorsal artery of both feet was weak. Astilbin 500mg was taken twice, 4 weeks as a course of treatment. After taking the medicine, the symptoms of diabetic foot gangrene were significantly improved, and he basically recovered after continuing to take the medicine for two months, and there was no recurrence in the follow-up for two years.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com