Somatostatin powder injection composition and preparation process thereof
A technology of somatostatin and preparation process, which is applied in the field of medicine, can solve the problems of polluting liquid medicine, easily producing glass shavings, and the existence of safety and quality risks, and achieve the effects of energy saving, good moldability of finished products, and accelerated water sublimation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
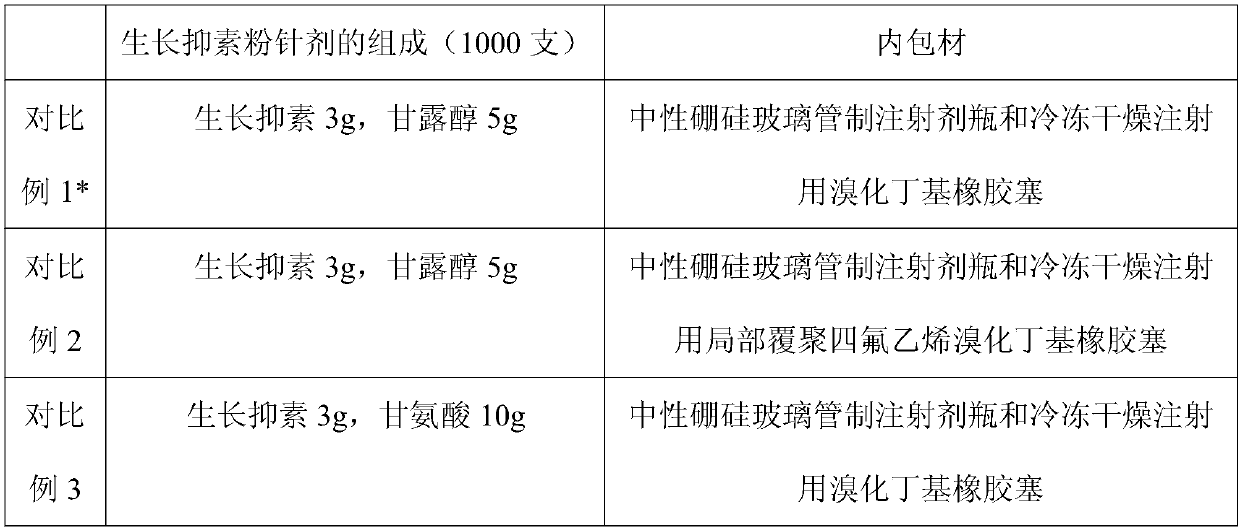
[0028] A somatostatin powder injection composition, prescription:
[0029] Somatostatin 0.25g
[0030] Glycine 10g
[0031] Add water for injection to 1000ml to make 1000 sticks.
[0032] A preparation process of somatostatin powder injection composition:
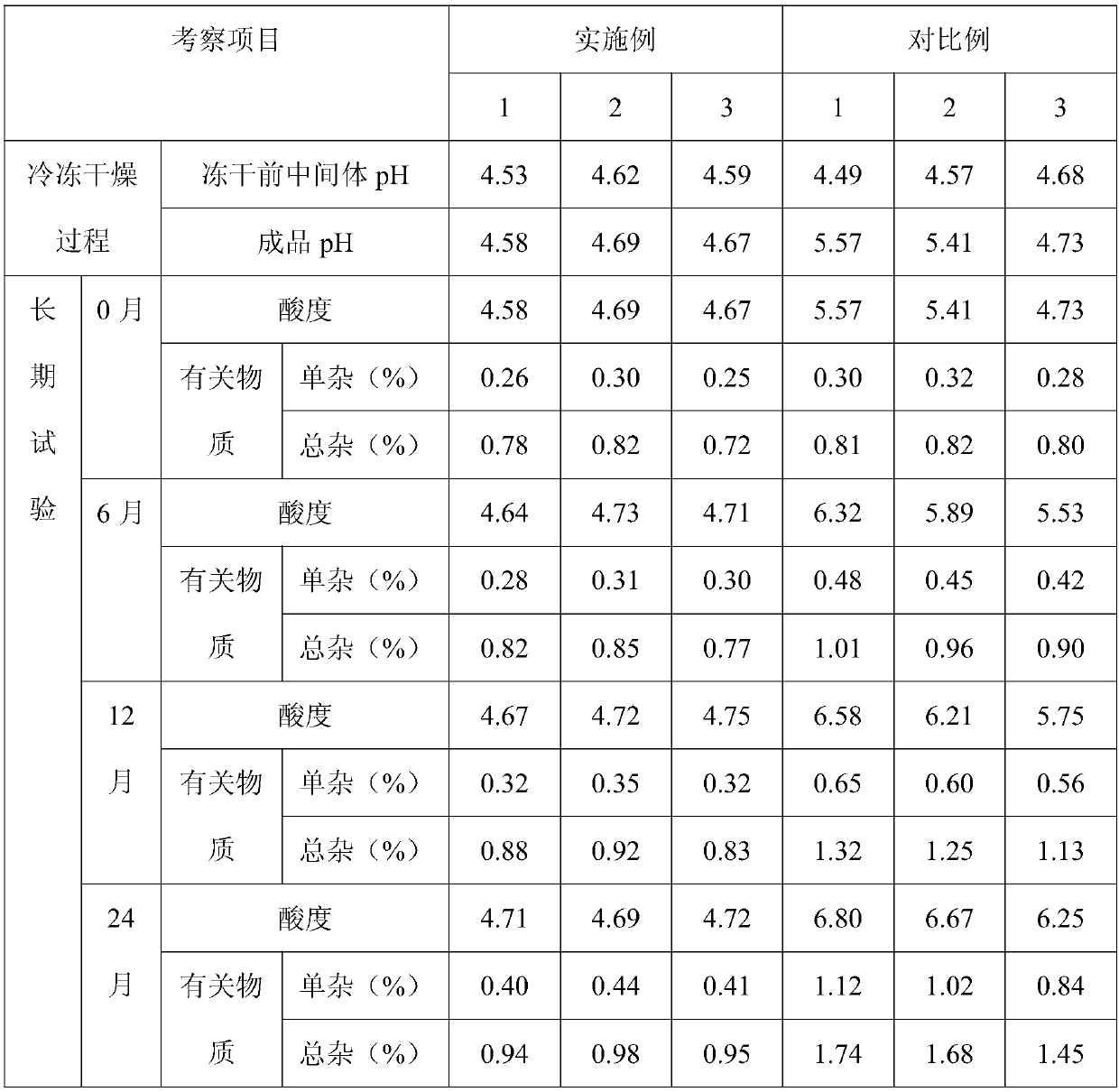
[0033] (1) Weigh the prescribed amount of somatostatin and glycine into a liquid preparation tank, add 80% water for injection and stir to dissolve, measure the pH value, adjust the pH to 3.5-5.5 with 1mol / L acetic acid solution.
[0034] (2) Add 0.05% (w / v) activated carbon and stir for 30 minutes, filter and decarbonize.
[0035] (3) Add water for injection to the full amount, stir evenly, and take the intermediate for testing.
[0036] (4) After passing the inspection of the intermediate, it is filtered through a 0.22 μm polyethersulfone filter element, packed in 2ml neutral borosilicate glass control injection bottles, and partially covered with polytetrafluoroethylene membrane brominated butyl rubber stopper for fr...
Embodiment 2
[0039] A somatostatin powder injection composition, prescription:
[0040] Somatostatin 0.75g
[0041] Glycine 10g
[0042] Add water for injection to 1000ml to make 1000 sticks.
[0043] A preparation process of somatostatin powder injection composition:
[0044] (1) Weigh the prescribed amount of somatostatin and glycine into a liquid preparation tank, add 80% water for injection and stir to dissolve, measure the pH value, adjust the pH to 3.5-5.5 with 1mol / L acetic acid solution.
[0045] (2) Add 0.05% (w / v) activated carbon and stir for 30 minutes, filter and decarbonize.
[0046] (3) Add water for injection to the full amount, stir evenly, and take the intermediate for detection.
[0047] (4) After passing the inspection of the intermediate, it is filtered through a 0.22 μm polyethersulfone filter element, packed in 2ml neutral borosilicate glass control injection bottles, and partially covered with polytetrafluoroethylene membrane brominated butyl rubber stopper for ...
Embodiment 3
[0050] A somatostatin powder injection composition, prescription:
[0051] Somatostatin 3.0g
[0052] Glycine 10g
[0053] Add water for injection to 1000ml to make 1000 sticks.
[0054] A preparation process of somatostatin powder injection composition:
[0055] (1) Weigh the prescribed amount of somatostatin and glycine into a liquid preparation tank, add 80% water for injection and stir to dissolve, measure the pH value, adjust the pH to 3.5-5.5 with 1mol / L acetic acid solution.
[0056] (2) Add 0.05% (w / v) activated carbon and stir for 30 minutes, filter and decarbonize.
[0057] (3) Add water for injection to the full amount, stir evenly, and take the intermediate for detection.
[0058] (4) After passing the inspection of the intermediate, it is filtered through a 0.22 μm polyethersulfone filter element, packed in 2ml neutral borosilicate glass control injection bottles, and partially covered with polytetrafluoroethylene membrane brominated butyl rubber stopper for f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com