Preparation method of cabozantinib s-malate tablets
A technology of cabozantinib malate tablets and cabozantinib malate, which is applied in the field of medicine, can solve problems such as poor fluidity, and achieve the effect of less process steps, suitable for industrial production, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 powder direct compression method prepares cabozantinib malate tablet
[0047] Take the raw and auxiliary materials (5000 tablets) according to the following prescription:
[0048] prescription
Content per piece
Cabozantinib Malate
76.03mg
Microcrystalline Cellulose (PH102)
332.7mg
Lactose (F100)
300mg
30mg
Colloidal silica (A200P)
3.75mg
7.5mg
Core weight
750mg
Film coating premix (Opadry 03K92254) weight gain
4.0%
[0049] Preparation:
[0050] 1. Grind cabozantinib malate until D90≦25μm, set aside;
[0051] 2. Put cabozantinib malate, microcrystalline cellulose, lactose, carboxymethyl starch sodium, and colloidal silicon dioxide in a 10L hopper mixer machine. The materials account for 50% of the total volume of the hopper mixer. Turn on the equipment and mix After 150 revolutions, pass through a 60-mesh ...
Embodiment 2
[0056] Example 2 Preparation of cabozantinib malate tablets by wet granulation and tabletting
[0057] Take the raw and auxiliary materials (5000 tablets) according to the following prescription:
[0058] prescription
Content per piece
Cabozantinib Malate
76.03mg
Microcrystalline Cellulose (PH102)
93.24mg
46.61mg
Hydroxypropyl Cellulose (EXF)
7.2mg
Colloidal silica (A200P)
0.72mg
14.4mg
1.8mg
Core weight
210mg
Film coating premix (Opadry 03K92254) weight gain
4.0%
[0059] Preparation:
[0060] 1. Grind cabozantinib malate until D90≦25μm, set aside;
[0061] 2. Put cabozantinib malate, microcrystalline cellulose, lactose, and croscarmellose sodium (internal addition) into the wet granulator, turn on the equipment, and pre-mix for 20 minutes;
[0062] 3. Add an appropriate amount of water and stir ...
Embodiment 3
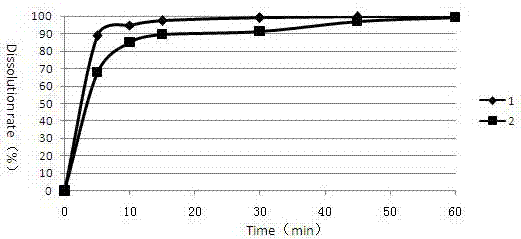
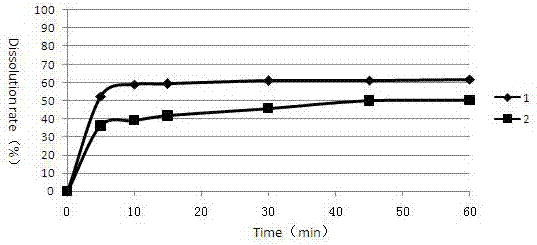
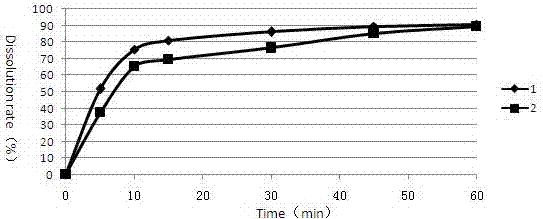
[0071] Example 3 Cabozantinib Malate Tablets (prepared in Examples 1 and 2) Take 0.01N HCl (0.5% Triton) as the Dissolution Profile Determination of Dissolution Medium
[0072] Determination of the dissolution curve of cabozantinib malate tablets in pH4.5 acetic acid-sodium acetate buffer solution (0.5% Triton) dissolution medium as follows:
[0073] Get respectively the cabozantinib malate sheet prepared by embodiment 1 and 2 according to the dissolution assay method (the second method of the second appendix XC of the Chinese Pharmacopoeia version in 2010), with water pH4.5 acetic acid-sodium acetate buffer solution (0.5% Triton) is the dissolution medium, and the rotating speed is 75 revolutions per minute, and it is operated according to the law. At 5, 10, 15, 30, 45, and 60 minutes, take 3.0ml of the dissolution solution, filter it through a 0.45μm filter membrane, and add the same amount of dissolution medium at the same temperature at the same time. The dissolution resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com