Clinical stable eye drops
A stable, eye-drop technology, applied in the direction of topical antibacterial agents, medical preparations containing active ingredients, organic active ingredients, etc. It is not easy to settle and stratify, the suspension system is stable, and the corrosion resistance is enhanced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following clearly and completely describes the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are only some of the embodiments of the present invention, but not all of them. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
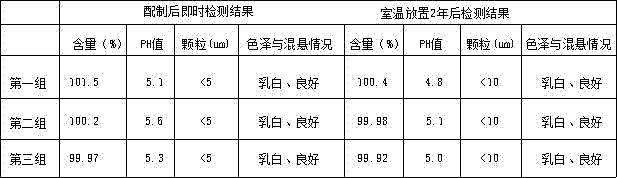
[0018] A clinical stable eye drop, comprising a formula by weight: 14.3 parts by weight of hydrocortisone acetate, 57.1 parts by weight of boric acid, 2.3 parts by weight of Tween-80, 0.68 parts by weight of nitrobenzene Mercury, 11.4 parts by weight of sodium carboxymethylcellulose, 2.8 parts by weight of methylcellulose, 10.8 parts by weight of polyhexamethylene biguanide hydrochloride, and 0.62 parts by weight of additives.
[0019] The preparation method of described clinical stable eye drop comprises the following steps:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com