Two kinds of ACE inhibitory peptides derived from Larimichthys crocea connetin
A technology for inhibiting peptides and large yellow croakers, applied in peptide/protein ingredients, protein-containing food ingredients, medical preparations containing active ingredients, etc., can solve the problems of long time-consuming and high cost, and achieve reduced quantity and high adjuvant treatment Blood pressure, the effect of increasing the probability of successful screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Selection of raw materials
[0024] Search for protein sequences related to large yellow croaker from the NCBI database for analysis, and the selection is based on the availability of sequence information of the parent protein and an important component of the large yellow croaker muscle protein. The final selection of the large yellow croaker Titin amino acid (ACCESSION: KKF25250), the amino acid number is 17943.
Embodiment 2 3
[0025] Example 2 Screening and activity determination of tripeptides
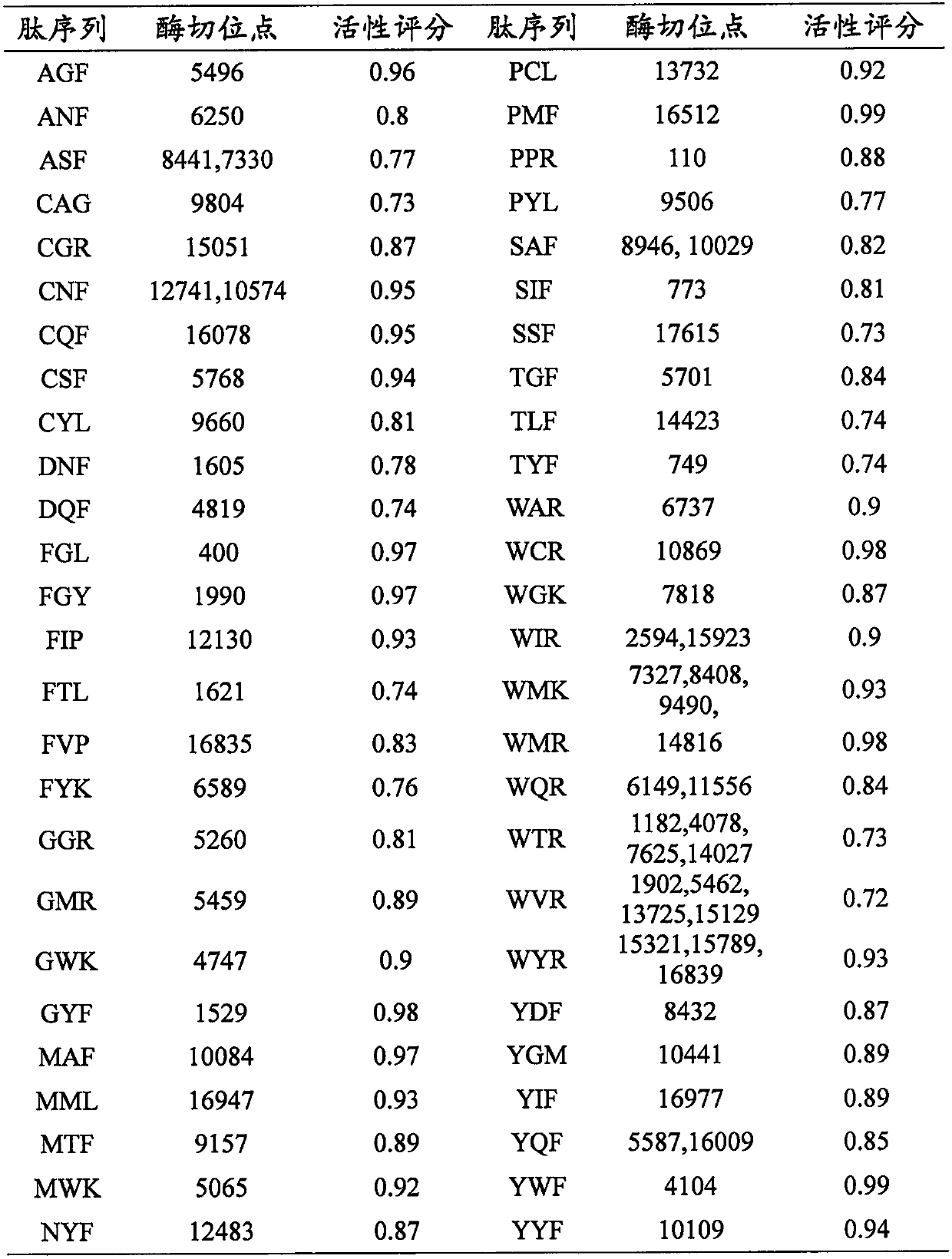
[0026] Pepsin (pH 1.3) and trypsin were used to virtually hydrolyze the troponin by ExPASy PeptideCutter, all tripeptides were selected, and the activity score was performed in PeptideRanker, and the 50 tripeptides with high scores were selected.
[0027] Use online tools Innovagen, ToxinPred and admetSAR to predict water solubility, toxicity and ADMET properties, respectively. A non-toxic, good water-soluble tripeptide was selected. In ADMET prediction, it focuses on the determination of good intestinal absorption (HIA+) and the tripeptide (could penetrate the Blood-Brain Barrier, BBB+) that can penetrate the Blood-Brain Barrier (BBB+) as potential high-efficiency ACE inhibitor peptide candidates. Finally selected 8 required tripeptide sequences, docked with ACE (PDB ID: 1A1B) in SwissDock, and selected the two tripeptides WAR and WQR with the lowest docking energy value, with ΔG of -10.40 and -9.57kcal, respe...
Embodiment 3
[0033] Example 3 Verification of in vitro activity
[0034] The ACE inhibitory activity of WAR and WQR was verified by high performance liquid chromatography. Take the hippuryl histidine leucine (HHL) substrate solution, add the inhibitor and mix well, preheat it in a constant temperature water bath at 37°C for 3 to 5 minutes, then add the ACE solution to mix well, keep it at 37°C for 30 minutes, then add The reaction was terminated with 1mol / L HCl to obtain a reaction solution. At the same time, boric acid buffer was used instead of inhibitor solution as a blank control group. The reaction solution was directly analyzed by the HPLC system.
[0035] Chromatographic conditions: column temperature 25°C, flow rate 0.5mL / min, mobile phase acetonitrile: water 25:75 isocratic elution, detection wavelength 228nm.
[0036] The in vitro ACE inhibitory activities of WAR and WQR were verified through experiments, which were 31.17±0.79 and 231.33±0.02μM, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com