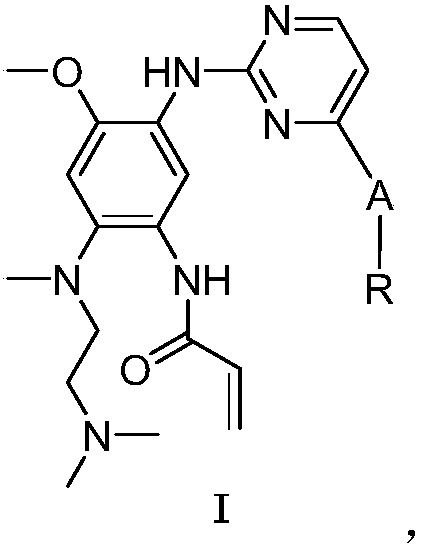
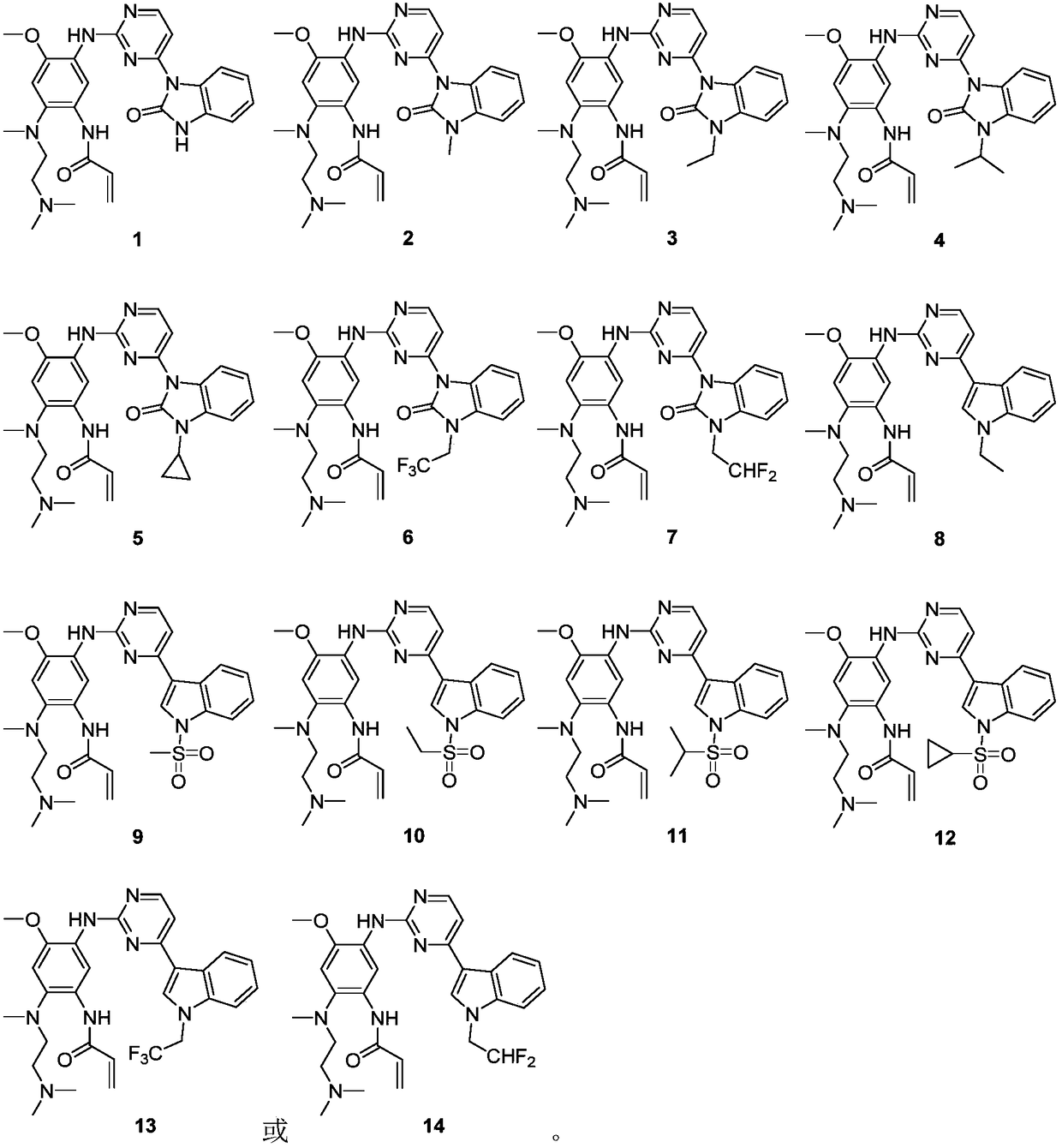
Solid pharmaceutical composition of EGFR inhibitor
A composition and drug technology, applied in the field of pharmacy, can solve the problems of low bioavailability, affecting the therapeutic effect of such compounds, unfavorable dissolution and absorption process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
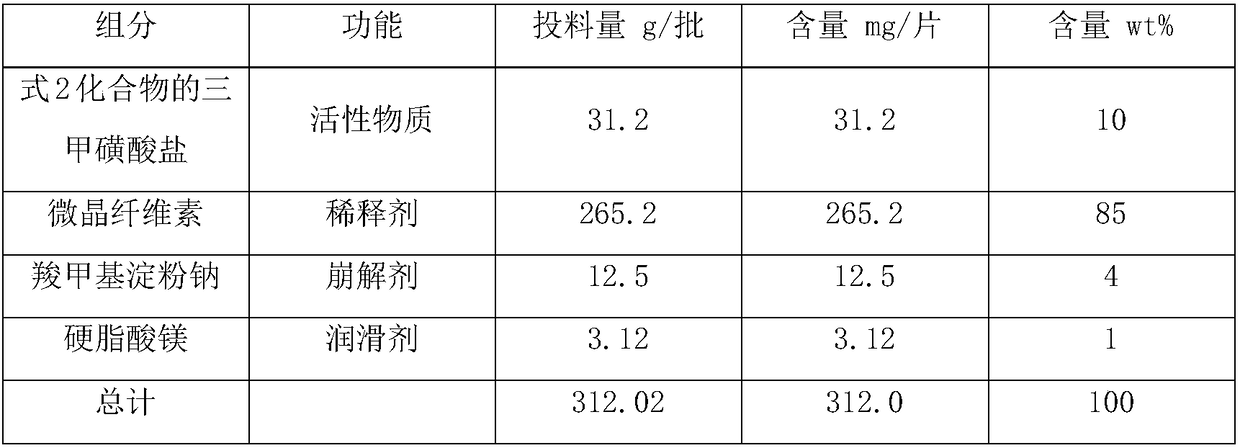
[0044] The trimesylate salt of the compound of formula 2 is crushed by airflow, and sieved; the microcrystalline cellulose is crushed by airflow, and sieved; Sodium starch and magnesium stearate were added in the mixer hopper, mixed for 30 minutes, and the tablet machine carried out dry compression to form tablets. The feeding amount of the material is 1000 tablets, each tablet is equivalent to containing 20mg of the compound of formula 2, and the composition of the prescription is as follows:
[0045] The prescription composition of the trimesylate tablet A of the compound of formula 2 in table 1
[0046]
Embodiment 2~ Embodiment 9
[0048] With reference to the preparation method of Example 1, the following tablets of Examples 2 to 9 were prepared:
Embodiment 2
[0050] The prescription composition of the trimesylate tablet B of the compound of table 2 formula 2
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com