Senecio scandens derivative and application thereof in drugs
A derivative, sensible technology, applied in the direction of organic chemistry, organic chemical methods, antiviral agents, etc., can solve problems such as easy to cause asthma and chronic obstructive pneumonia, use restrictions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
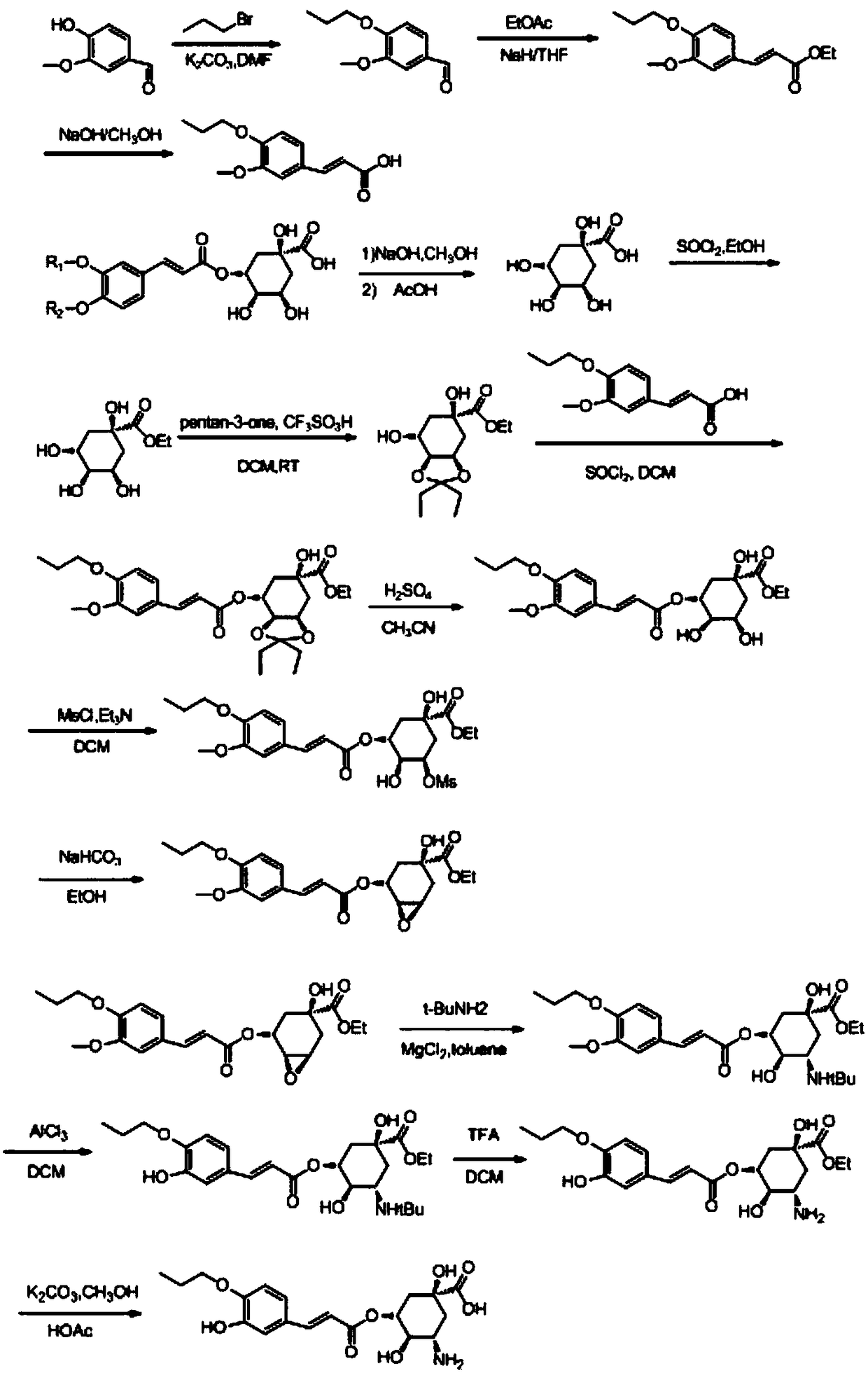
[0033] (1S, 3S, 4R, 5R)-3-amino-1,4-dihydroxy-5-(((E)-3-(3-hydroxy-4-propoxyphenyl)acryloyl)oxy) The synthesis of cyclohexanecarboxylic acid comprises the following steps:
[0034] Synthesis of S1, 3-methoxy-4-n-propoxybenzaldehyde: 4-hydroxyl-3-methoxybenzaldehyde (15.2g, 100mmol) was dissolved in 300mL of DMF, bromopropane (13.4 g, 110mmol) and K 2 CO 3 (15.2 g, 110 mmol). After the reaction solution was reacted at 100° C. for 6 hours, TLC (PE:EA=2:1) monitored that the reaction was complete. The reaction solution was poured into water (500 mL), and extracted with ethyl acetate (500 mL×3). The combined reactions were sequentially washed with saturated NH 4 Cl aqueous solution (500mL×3), water (300mL) and saturated brine (300mL) were washed, dried over anhydrous sodium sulfate, filtered, and concentrated to give a yellow oily product (15.3g, 79%);
[0035] S2. Synthesis of (E)-3-(3-methoxy-4-propoxyphenyl)ethyl acrylate: NaH (0.26g, 11mmol) was dissolved in 20mL of TH...
Embodiment 2
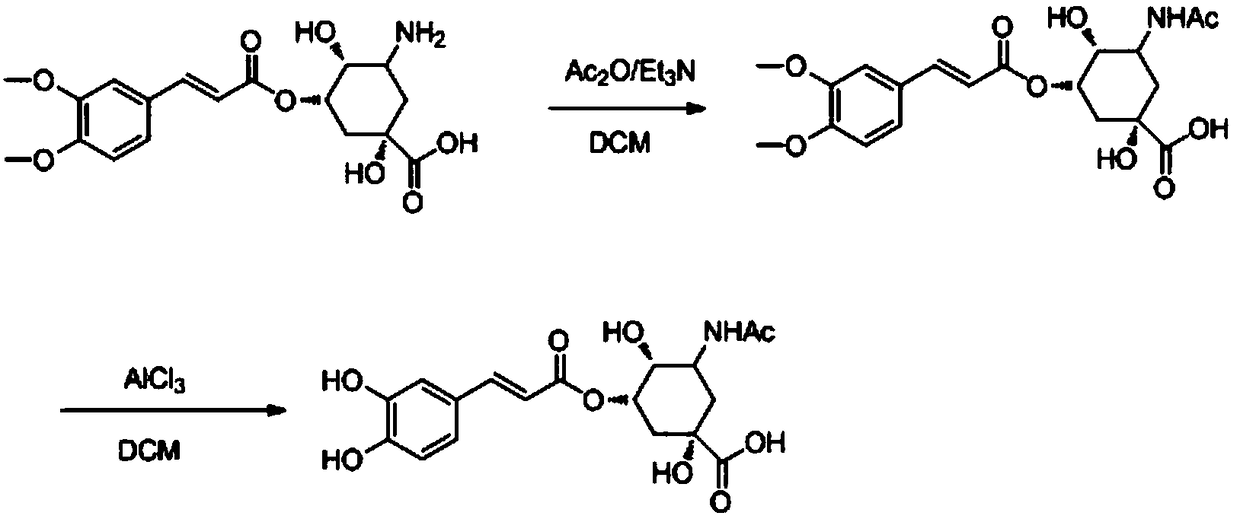
[0053] (1S, 3S, 4R, 5R)-3-amino-1,4-dihydroxy-5-(((E)-3-(3,4-di-hydroxyphenyl)acryloyl)oxy)cyclohexyl For the synthesis of alkanoic acid, referring to Example 1, TJB-002 was obtained by using 3,4-dihydroxybenzaldehyde as the starting material.
[0054] MS(ESI,pos.ion)m / z:380(M+1);
[0055] 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 10.5(s,1H), 7.48(s,1H), 6.89-7.25(m,3H), 6.31(s,1H), 5.33(s,1H), 5.12(dd,J=11.2 ,2H), 43(t,J=7.2Hz,2H),4.05(m,4H),3.58(S,1H),3.55(s,1H),1.93-2.65(m,5H);
[0056] 13 C NMR (101MHz, DMSO-d 6 )δ (ppm): 177.44, 167.04, 149.60, 146.23, 143.2, 128.6, 123.6, 116.8, 114.6, 112.5, 81.5, 79.4, 68.9, 64.4, 64.4, 47.5, 38.3, 35.6.
Embodiment 3
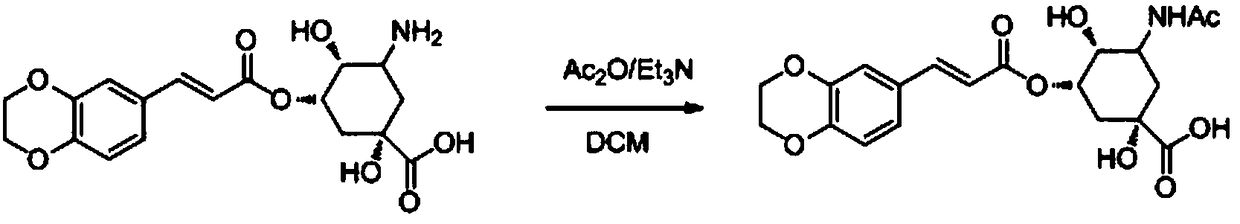
[0058] (1S, 4R, 5S)-3-amino-5-(((E)-3-(3,4-dihydroxyphenyl)acryloyl)oxy)-1,4-dihydroxycyclohexanecarboxylic acid Synthesis Referring to Example 1, TJB-003 was prepared by using 3,4-dimethoxybenzaldehyde as the starting material.
[0059] MS(ESI,pos.ion)m / z:354(M+1);
[0060] 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 10.5 (s, 1H), 7.48 (s, 1H), 6.82-7.28 (m, 3H), 6.36 (s, 1H), 5.38 (s, 1H), 5.15 (dd, J = 11.2 ,2H), 4.01(m,2H), 3.58(S,1H), 3.66(s,1H), 1.73-2.68(m,5H);
[0061] 13 C NMR (101MHz, DMSO-d 6 )δ (ppm): 176.54, 166.14, 149.20, 147.26, 144.3, 127.7, 122.7, 116.7, 114.6, 112.3, 81.6, 79.1, 68.4, 47.5, 38.3, 35.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com