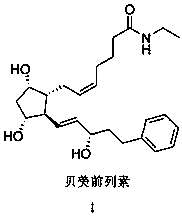
Method for purifying bimatoprost
A bimetrexed and purification method technology, applied in the field of preparing high-purity bimetrexed, can solve the problems of high cost, unfavorable production scale-up, complicated operation and the like, achieves mild conditions, simple and convenient operation, and overcomes the complicated process of removing impurities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
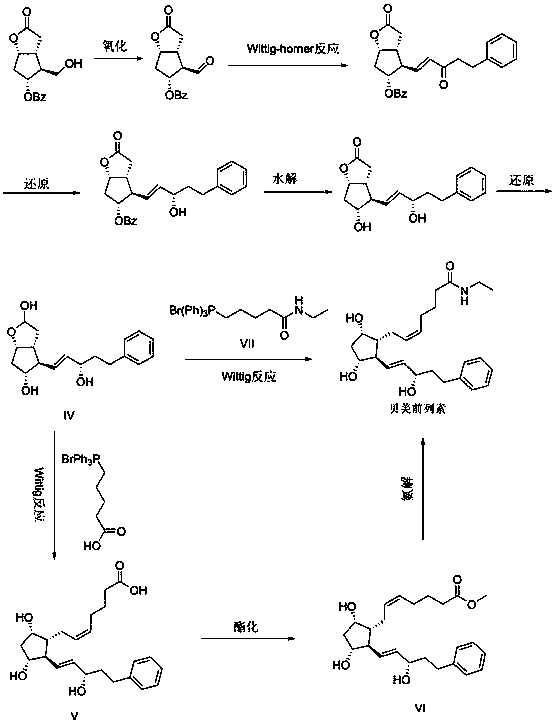
[0031] Preparation of Crude Bimatoprost
[0032] Compound IV was prepared according to the method described in EP2495235, and compound VII was prepared according to the method described in Org. Lett., 2015, 17(3), pp 504-507.
[0033] Add 37.8g (3.5eq) of compound V to the reaction flask, add 50mL of tetrahydrofuran, stir evenly and cool down to -10°C, dissolve 18g (7eq) of potassium tert-butoxide in 50mL of tetrahydrofuran and add it dropwise to the reaction solution, controlling the temperature not to exceed -10°C, after adding and keeping warm for 30min, dissolve 7g (1eq) of compound VII in 50mL of tetrahydrofuran, add dropwise to the reaction solution, return to room temperature for about 2h after adding, TLC (EA / MeOH=100:5) to monitor the completion of the reaction, go to Add 200mL saturated ammonium chloride solution to the reaction solution, stir to dissolve and separate the liquids, extract the aqueous phase with 200mL ethyl acetate, combine the organic phases, wash wi...
Embodiment 2
[0035] Preparation of tert-butyldimethyl bimatoprost
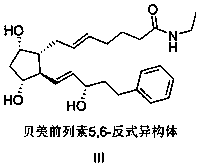
[0036] Dissolve 9g (1eq) of crude bimatoprost in 90mL of dichloromethane, add 13.0g (4eq) of TBSCl, 6.6g (4.5eq) of imidazole, stir and react overnight, add 90mL of saturated ammonium chloride solution to the reaction solution, divide liquid, the organic phase was washed with 90mL saturated brine, dried with 50g of anhydrous sodium sulfate, and then concentrated to obtain about 18g of a yellow oil, which was chromatographed on a 500g 200-300 mesh silica gel column, eluent (n-heptane / ethyl acetate = 12:1) to obtain 15 g of tert-butyldimethyl bimatoprost, which was detected by HPLC with a purity of 99.92%, a yield of 91.5%, and a 5,6-trans isomer impurity content of 0.05%.
Embodiment 3
[0038] Preparation of tert-butyldiphenyl bimatoprost
[0039] Dissolve 9g (1eq) crude bimatoprost in 90mL dichloromethane, add 23.8g (4eq) TBDPSCl, 6.6g (4.5eq) imidazole, stir and react overnight, add 90mL saturated ammonium chloride solution to the reaction solution, divide liquid, the organic phase was washed with 90mL saturated brine, dried with 50g of anhydrous sodium sulfate, and then concentrated to obtain about 30g of a yellow oil, which was chromatographed on a 600g 200-300 mesh silica gel column, eluent (n-heptane / ethyl acetate=12 :1), to obtain 23 g of tert-butyldiphenyl bimatoprost, yield: 94.3%, HPLC detection, purity 99.91%, 5,6-trans isomer impurity content 0.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com