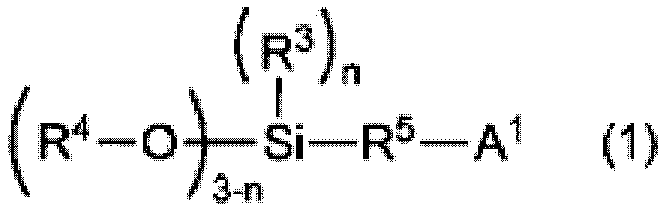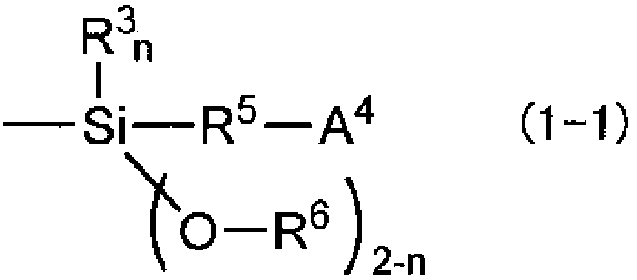Pneumatic tire
一种充气轮胎、氢化共聚物的技术,应用在特殊轮胎、轮胎零部件、运输和包装等方向,能够解决难以同时提高燃料经济性和耐磨性、燃料经济性相悖耐磨性等问题,达到良好燃料经济性、耐磨性和加工性均衡提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0138] The present invention is specifically described with reference to but not limited to the following examples.
[0139] The chemicals used in the synthesis or polymerization are shown below. Chemicals were purified as necessary by conventional techniques.
[0140] n-Hexane: manufactured by Kanto Chemical Co., Ltd.
[0141] Styrene: a product manufactured by Kanto Chemical Co., Ltd.
[0142] Butadiene: 1,3-butadiene manufactured by Tokyo Chemical Industry Co., Ltd.
[0143] THF: Tetrahydrofuran manufactured by Kanto Chemical Co., Ltd.
[0144] TMEDA: N,N,N',N'-tetramethylethylenediamine manufactured by Kanto Chemical Co., Ltd.
[0145] n-butyllithium solution: 1.6M hexane solution of n-butyllithium manufactured by Kanto Chemical Co., Ltd.
[0146] Ethanol: a product manufactured by Kanto Chemical Co., Ltd.
[0147] 2,6-di-tert-butyl-p-cresol: Nucrac 200 manufactured by Ouchi Shinko Chemical Industry Co., Ltd.
[0148] Amine modifier: N,N-bis(trimethylsilyl)-aminopro...
Synthetic example 1
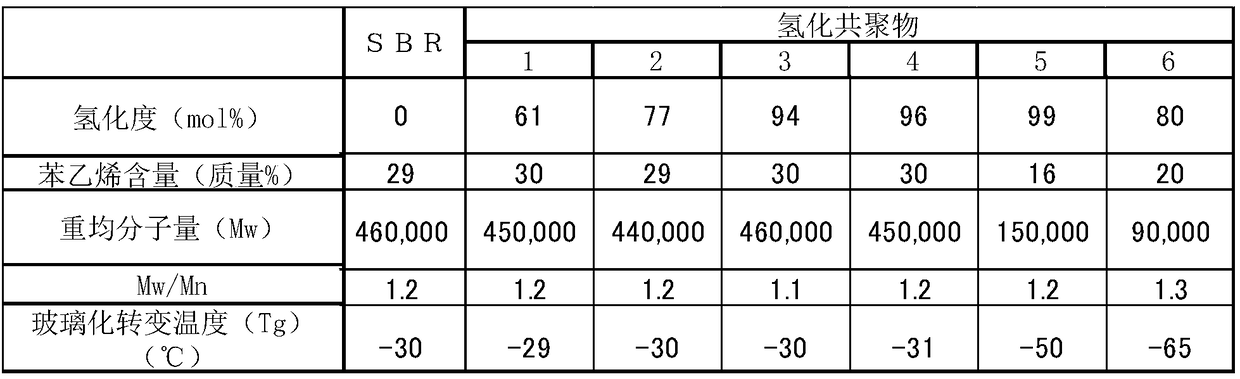
[0159] Synthesis Example 1 (Synthesis of SBR)
[0160] 2000 mL of n-hexane, 60 g of styrene, 140 g of butadiene, 2.5 g of THF and 0.45 mmol of n-butyllithium were added to a heat-resistant reaction vessel fully purged with nitrogen, followed by stirring at 50 °C for 5 h to induce polymerization. After the reaction was terminated by adding ethanol, 1 g of 2,6-di-tert-butyl-p-cresol was added to the reaction solution. The resulting solution was purified by reprecipitation to obtain the polymer (SBR).
Synthetic example 2
[0161] Synthesis Example 2 (Synthesis of Hydrogenated Copolymer 1)
[0162] A hydrogenated copolymer 1 was produced in the same manner as in Synthesis Example 1 except that the obtained polymer was hydrogenated. Specifically, after the polymerization conversion reaction in Synthesis Example 1, the polymerization reaction was not terminated by adding ethanol. Instead, the reaction solution was then stirred for 20 minutes while supplying hydrogen at a pressure of 0.4 MPa gauge to react unreacted polymer terminal lithium with hydrogen to form lithium hydride. Hydrogenation was carried out at a hydrogen supply pressure of 0.7 MPa gauge pressure and a reaction temperature of 90° C. using a titanocene-based dichloride catalyst. Once the cumulative amount of absorbed hydrogen reached an amount corresponding to the target degree of hydrogenation, the reaction temperature was returned to room temperature, and the hydrogen pressure was returned to normal pressure. Then, the reaction s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com