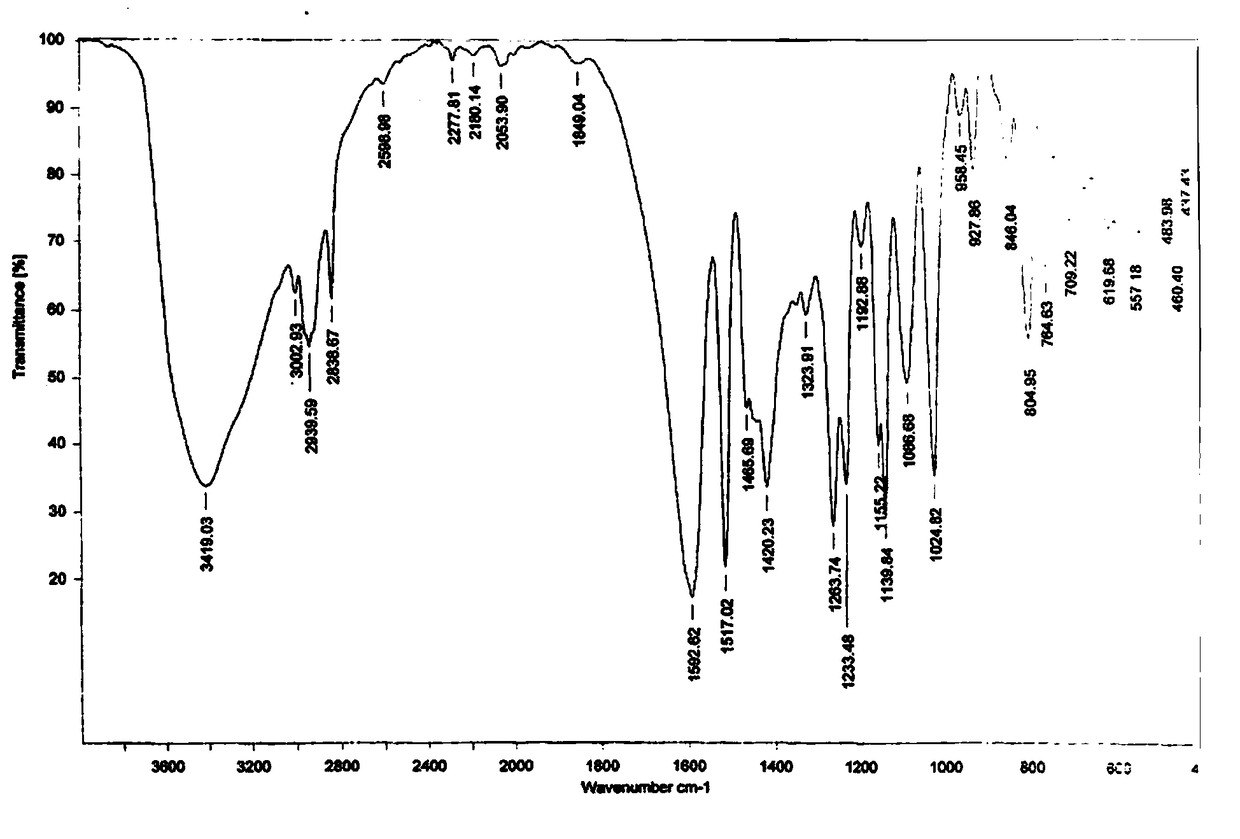
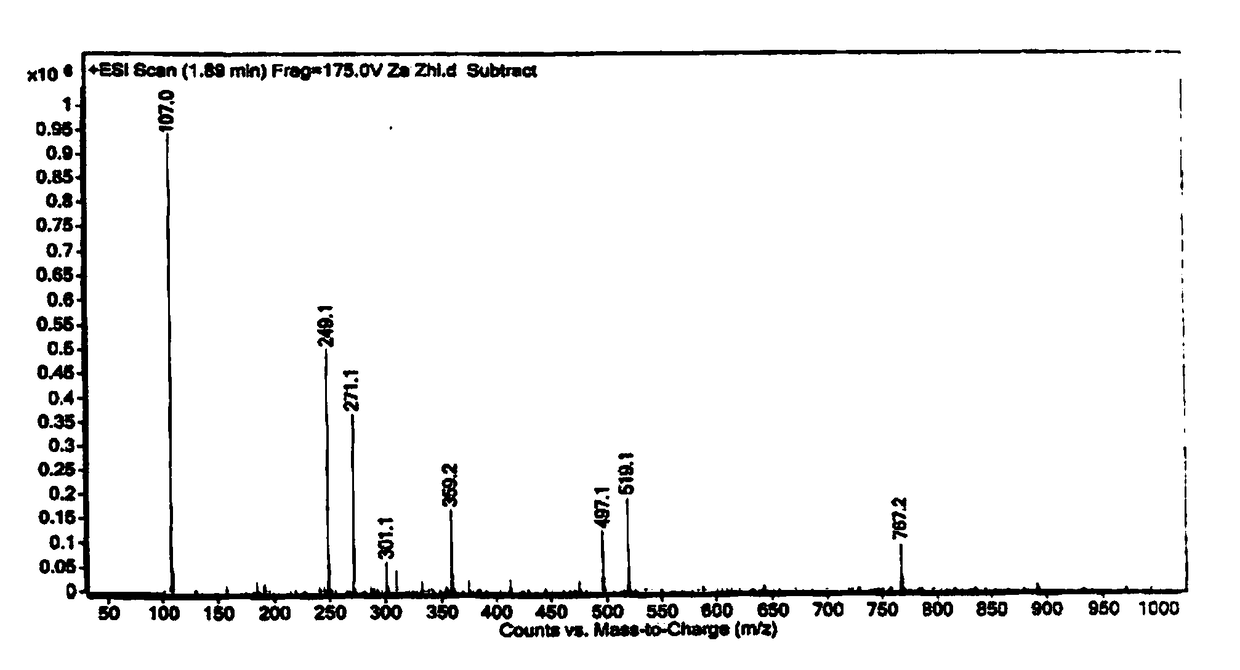
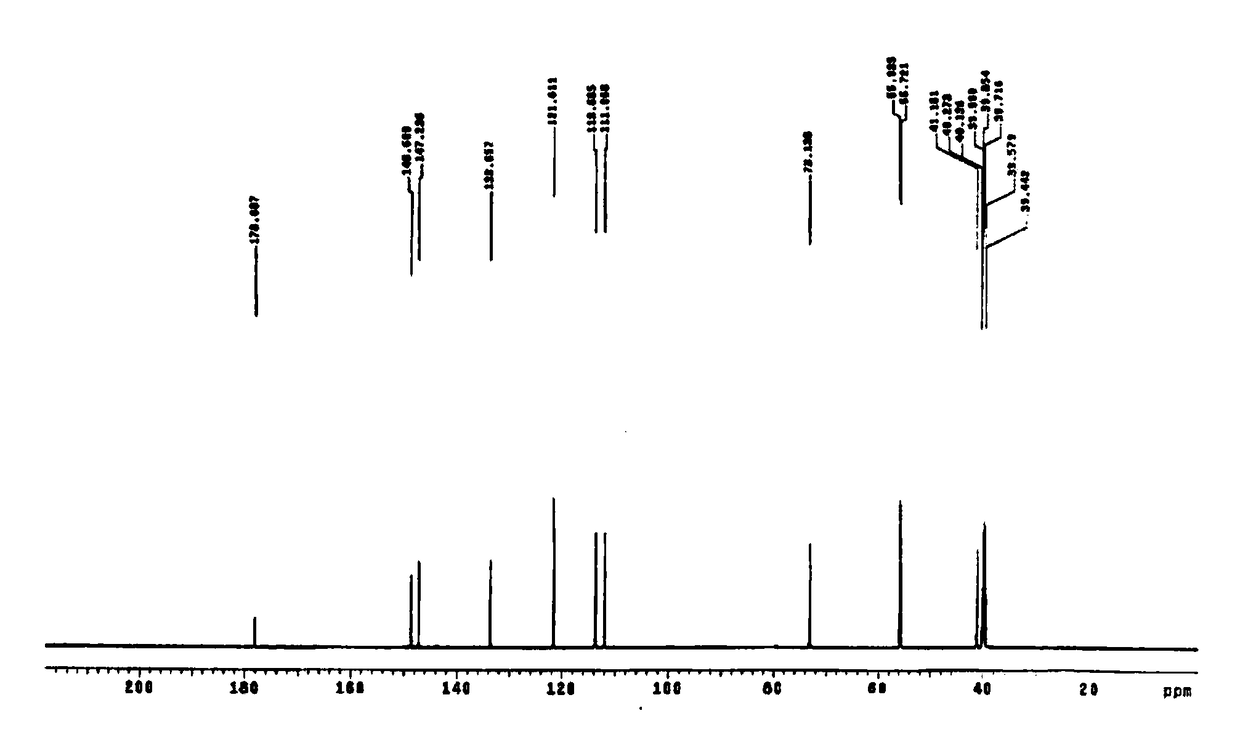
Synthesis method of 2-hydroxyl-3-(3,4-dimethoxy phenyl)propionic acid
A technology of dimethoxyphenyl and synthetic methods, applied in the field of synthesis of 2-hydroxy-3-propionic acid, can solve the difficult to meet the needs of impurity research, low purity of L-350 impurity A, difficult to handle waste, etc. problems, to achieve the effects of low toxicity, easy handling, and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 10g (0.04mol) of B to a 1L autoclave, 26.6g (0.2mol) of 30% liquid caustic soda, and 85mL of water, keep the temperature at 127.5±2.5°C, and react at a pressure of 0.3±0.1MPa for 5 hours, then concentrate under reduced pressure. Adjust the pH to 5 with hydrochloric acid. Cool down to 5°C, filter and wash with water to obtain 5.6 g of yellow crystals. The yield is 61.9%, the melting point is 103-105°C, and the purity is 99.4%.
Embodiment 2
[0031] Add 10g (0.04mol) of B to a 1L autoclave, 21.6g (0.12mol) of 30% sodium methoxide, and 80mL of methanol, keep the temperature at 65±5°C, and react at a pressure of 0.9±0.1MPa for 8 hours, then concentrate under reduced pressure. Adjust the pH to 5.5 with hydrochloric acid. Cool down to 6°C, filter and wash with water to obtain 7.2 g of yellow crystals. The yield is 79.5%, the melting point is 103-105°C, and the purity is 99.3%.
Embodiment 3
[0033] Add 10g (0.04mol) of B to a 1L autoclave, 60.4g (0.16mol) of 18% sodium ethoxide, and 50mL of ethanol, keep the temperature at 75±5°C, and react at a pressure of 0.7±0.1MPa for 12 hours, then concentrate under reduced pressure. Adjust the pH to 5.5 with hydrochloric acid. Cool down to 8°C, filter and wash with water to obtain 5.2 g of yellow crystals. Yield 57.5%. The melting point is 103-105°C, and the purity is 99.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com