Gene medicine for preventing and treating CNV (choroidal neovascularization)-associated eye diseases
A gene and virus technology, applied in the field of genetic medicine for the prevention and treatment of choroidal neovascularization-related eye diseases, can solve problems such as increased medical burden, risk of shedding, infectious endophthalmitis, retina, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Construction of plasmid vectors
[0046] (1) Construction of different expression units carrying reporter gene Gluc
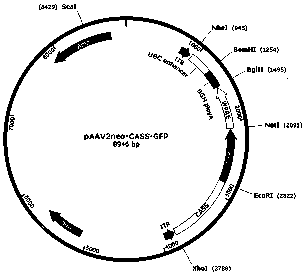
[0047] The promoter CASS (SEQ ID No.1), woodchuck hepatitis virus post-transcriptional regulatory element (WPRE, SEQ ID No.5) and UBC enhancer (SEQ ID No. .7), and introduced an XhoI restriction site at the 5' end of the CASS promoter, and an EcoRI restriction site at the 3' end. Through standard molecular biology operations, first replace the promoter on the AAV plasmid cloning vector pAAV2neo preserved in our laboratory with CASS, insert GFP and WPRE sequences between the EcoRI and BglII restriction sites of the multi-cloning site, and insert The UBC enhancer is introduced between the BamHI and NheI restriction sites downstream of BGH polyA, constituting the gene expression unit of the promoter-GFP-WPRE-poly A-UBC enhancer, named pAAV2neo-CASS-GFP, and its structure diagram is shown in figure 1 .
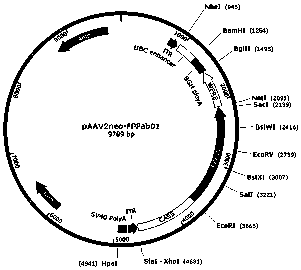
[0048] (2) Construction of AAV vector p...
Embodiment 2
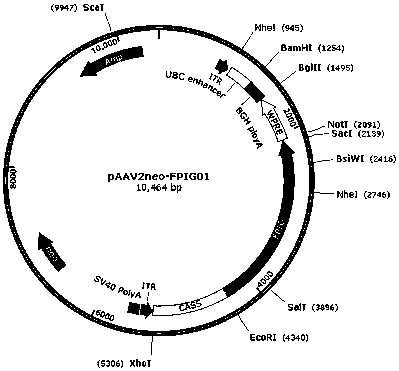
[0050] Example 2. Comparison of expression levels of pAAV2neo-FPFab01, pAAV2neo-FPIG01, pAAV2neo-FPFab02 and pAAV2neo-FPIG02 in cells
[0051] Transfect 293T and ARPE19 cells with pAAV2neo-FPFab01, pAAV2neo-FPIG01, pAAV2neo-FPFab02, pAAV2neo-FPIG02 and pAAV2neo-CASS-GFP, respectively, collect the cell supernatant 96 hours after transfection, and detect FPFab and FPIG in the supernatant by ELISA level of expression. The specific operation is briefly described as follows: 293T or ARPE19 cells were divided into 5×10 4 cells / well were seeded into 48-well culture plates at 37°C in 5% CO 2 Culture in an incubator until the cells are 80-90% confluent, and use lipofectamine 2000 to transfect the plasmids pAAV2neo-FPFab01, pAAV2neo-FPIG01, pAAV2neo-FPFab02, pAAV2neo-FPIG02 or pAAV2neo-CASS-GFP respectively. Set up 4 replicate holes. The supernatant was taken 96 hours after transfection, and the expression level of FPFab or FPIG in the supernatant was detected by ELISA method.
[00...
Embodiment 3
[0056] Example 3. Preparation and assay of recombinant AAV virus
[0057] The three-plasmid packaging system was used to package and purify the recombinant AAV virus. For specific operations, refer to literature [17]. Briefly, AAV vector plasmids (pAAV2neo-CASS-GFP, pAAV2neo-FPFab01 or pAAV2neo-FPIG01), helper plasmids (pHelper) and AAV Rep and Cap protein expression plasmids (pAAV-R2C5 or pAAV-R2C8 or pAAV-R2C9) were prepared according to After mixing at a molar ratio of 1:1:1, HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells and culture supernatant were harvested. According to the AAV vector purification method reported by Wu Xiaobing et al. [18], the virus titer was determined by dot blot method. For specific operations, refer to literature [19].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com