A method and automatic early warning system for production risk identification of pharmaceutical production enterprises
A risk identification and enterprise technology, applied in data processing applications, instruments, calculations, etc., can solve problems such as inability to efficiently use adverse reaction database resources, inability to push high-risk signals, lack of automatic early warning systems, etc., to reduce circulation risks and reduce Manpower, the effect of reducing the occurrence of major drug accidents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Risk identification of an injection production line of an enterprise in Guangdong Province
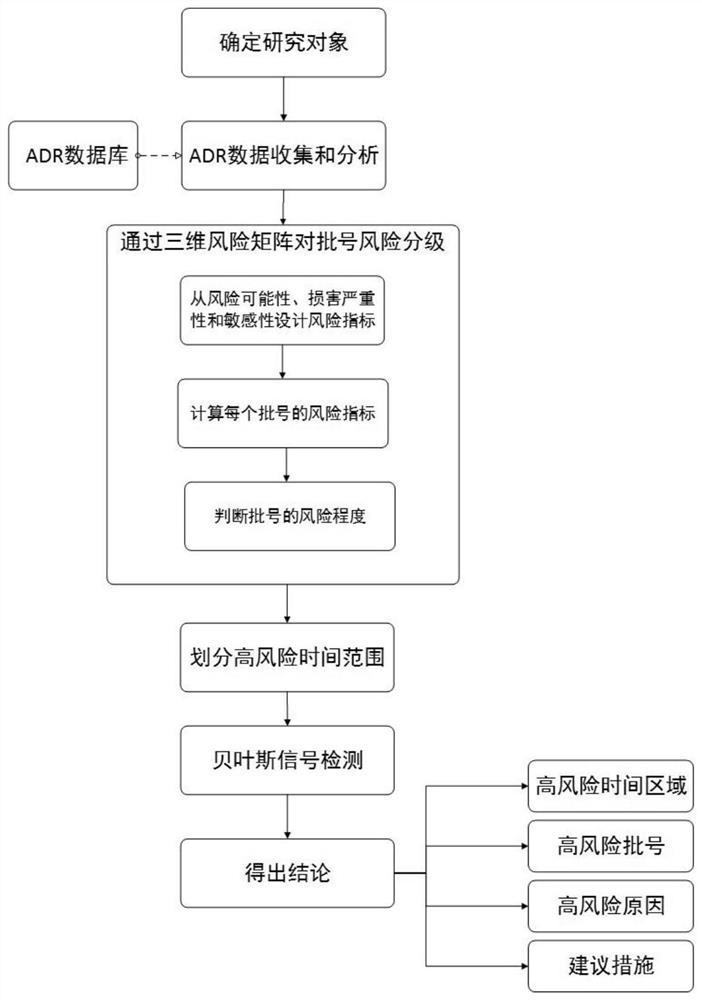
[0092] The specific methods and steps include:
[0093] 1. Research object ADR data collection and analysis: The research object is the injection production line of an enterprise in Guangdong Province. There are 6 types of injections, namely metronidazole sodium chloride injection, sodium chloride injection, glucose sodium chloride injection , glucose injection, tinidazole glucose injection and ofloxacin sodium chloride injection. From January 1, 2012 to August 30, 2015, the Guangdong Center received a total of 702 nationwide ADR reports involving 6 types of injections from an enterprise in Guangdong Province, involving a total of 504 batch numbers.
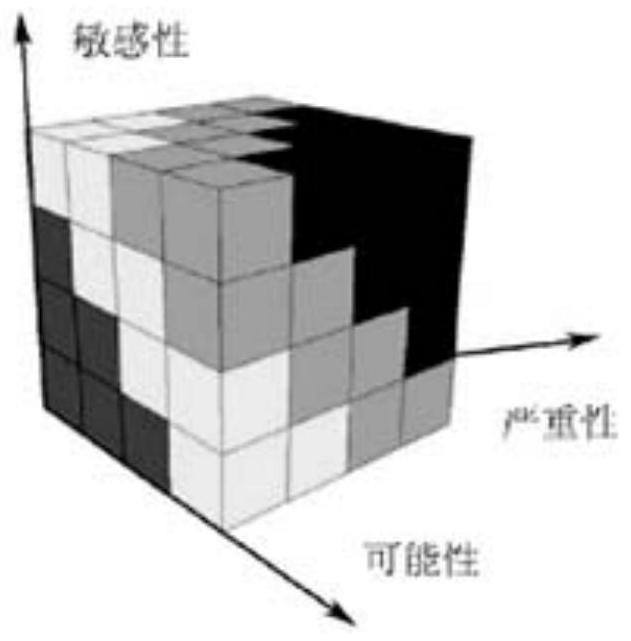
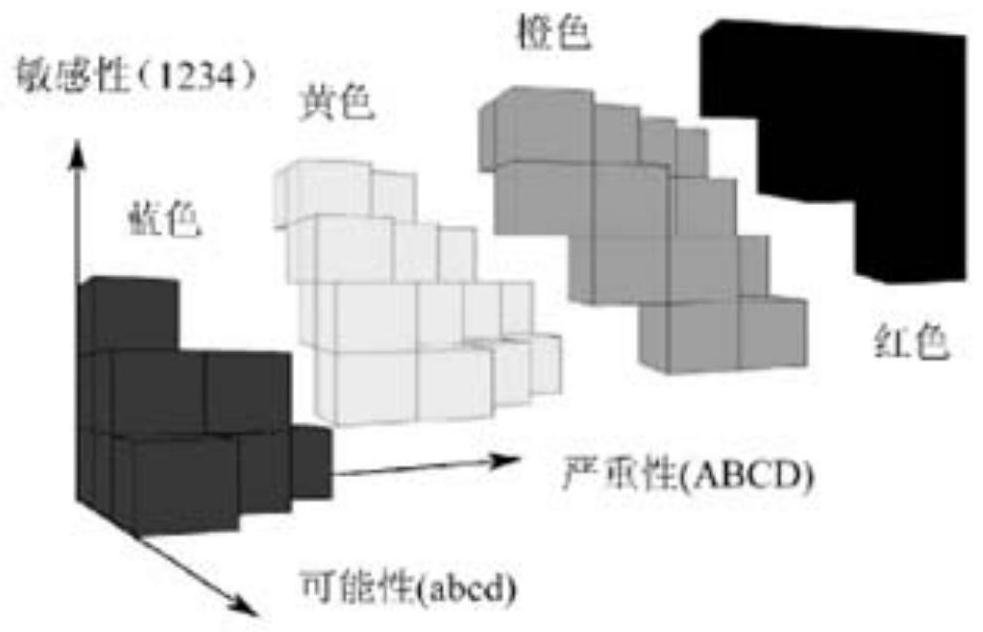
[0094] 2. Use the three-dimensional matrix method to carry out risk classification of batch numbers: realize the risk classification of batch numbers by establishing a three-dimensional risk matrix model.
[0095] (1) ...
Embodiment 2
[0112] Example 2: Using the risk automatic early warning system to carry out risk identification on the injection production line of an enterprise in Guangdong Province
[0113] The specific methods and steps include:
[0114] 1. Set the relevant information of the research enterprise: realize the information setting of the enterprise, production line, drug generic name, and batch number through the input module, and the execution steps are as follows:
[0115] (1) Enter the name of an enterprise in Guangdong Province in the system input module, and create a new research path for an enterprise in Guangdong Province;
[0116] (2) In the system, select "Injection" for the "Dosage Form" data item, and "Select All" for the "Drug Generic Name" data item.
[0117] 2. Capture the ADR report data of research companies: read the ADR reports involving research companies in the ADR database into the data capture module, classify them according to batch numbers, and save them in the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com