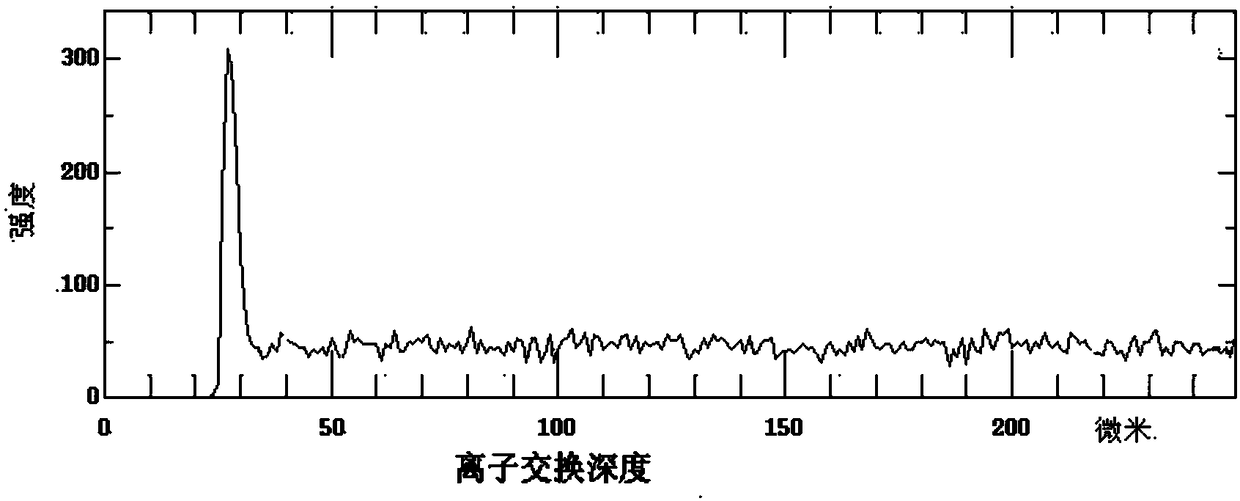
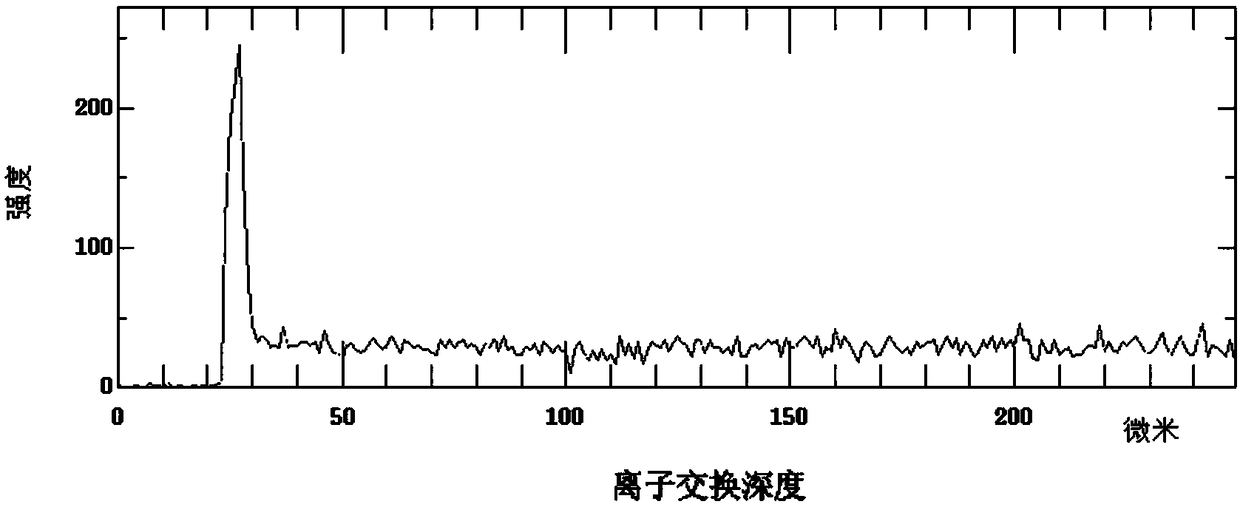
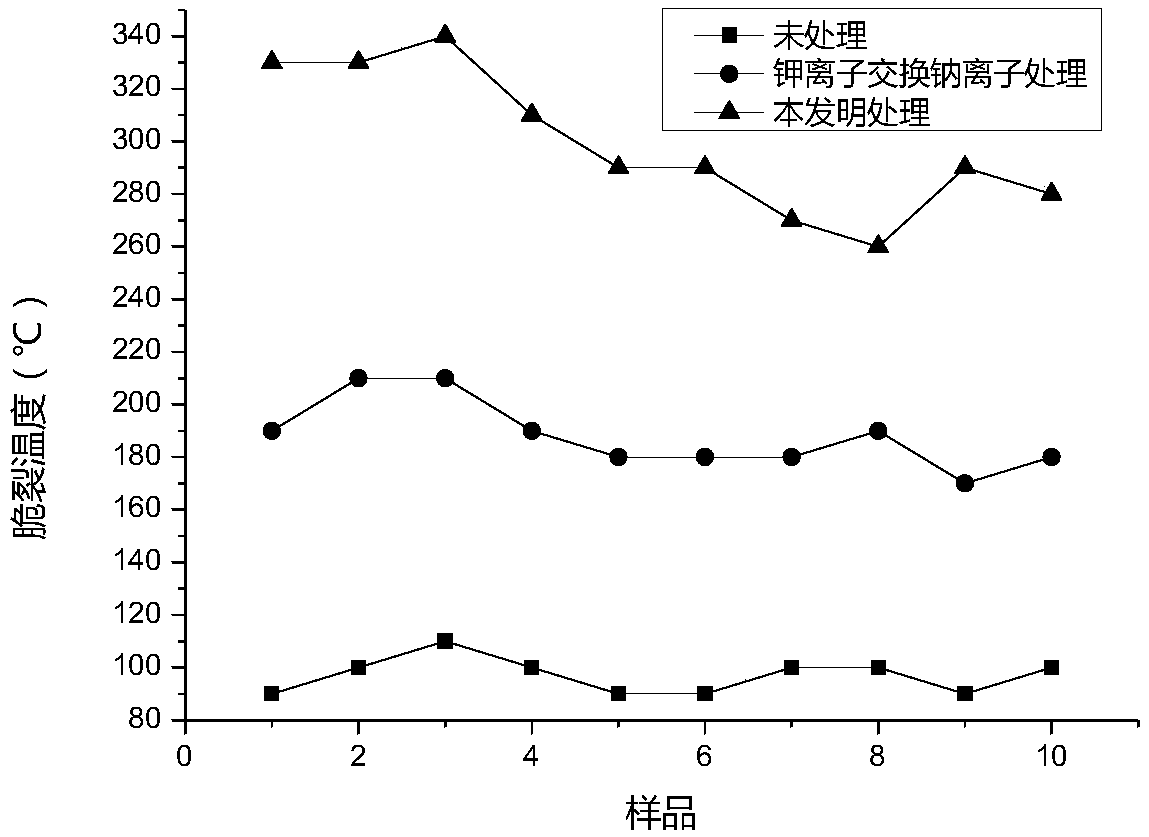
Ion exchange enhancement method of laser glass
A laser glass and ion exchange technology, applied in the field of laser glass, can solve the problems of reduced chemical stability of glass, easy to be corroded, and the surface cannot be effectively repaired, etc., to achieve enhanced chemical stability, long stress relaxation time, and improved surface chemical stability. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment adopts phosphate laser glass, and the compositions of mixed molten salt 1, mixed molten salt 2 and mixed acid are as follows:
[0041] Mix molten salt 1:
[0042] Composition wt%
[0043] RbNO3 10%
[0044] CsNO3 90%
[0045] Mix molten salt 2:
[0046] Composition wt%
[0047] LiNO3 15%
[0048] NaNO3 85%
[0049] Mixed acid:
[0050]
[0051] This embodiment includes the following steps:
[0052] S11: The sample is washed with deionized water and dried.
[0053] S12: Put the mixed molten salt 1 prepared in proportion into an ion exchange vessel, put it into a muffle furnace to raise the temperature to 450°C and stabilize it, then preheat the sample treated in step S11 to 340°C and transfer it to an ion exchange vessel.
[0054] S13: Take it out and anneal to room temperature in the annealing furnace after keeping it in the muffle furnace for 8 hours at 450°C.
[0055] S21: washing and drying the sample treated in step S13 with deionized wa...
Embodiment 2
[0062] This embodiment adopts phosphate laser glass, and the compositions of mixed molten salt 1, mixed molten salt 2 and mixed acid are as follows:
[0063] Mix molten salt 1:
[0064] Composition wt%
[0065] RbNO3 20%
[0066] CsNO3 80%
[0067] Mix molten salt 2:
[0068] Composition wt%
[0069] LiNO3 11%
[0070] NaNO3 89%
[0071] Mixed acid:
[0072]
[0073] This embodiment includes the following steps:
[0074] S11: The sample is washed with deionized water and dried.
[0075] S12: Put the mixed molten salt 1 prepared in proportion into an ion exchange vessel, put it into a muffle furnace to raise the temperature to 430°C and stabilize it, then preheat the sample treated in step S11 to 340°C and transfer it to an ion exchange vessel.
[0076] S13: Take it out and anneal to room temperature in the annealing furnace after keeping it in the muffle furnace for 10 hours at 430°C.
[0077] S21: washing and drying the sample treated in step S13 with deionized w...
Embodiment 3
[0084] This embodiment adopts phosphate laser glass, and the compositions of mixed molten salt 1, mixed molten salt 2 and mixed acid are as follows:
[0085] Mix molten salt 1:
[0086] Composition wt%
[0087] RbNO3 30%
[0088] CsNO3 70%
[0089] Mix molten salt 2:
[0090] Composition wt%
[0091] LiNO3 8%
[0092] NaNO3 92%
[0093] Mixed acid:
[0094]
[0095] This embodiment includes the following steps:
[0096] S11: The sample is washed with deionized water and dried.
[0097] S12: Put the mixed molten salt 1 prepared in proportion into an ion exchange vessel, put it into a muffle furnace to raise the temperature to 420°C and stabilize it, then preheat the sample treated in step S11 to 300°C and transfer it to an ion exchange vessel.
[0098] S13: Take it out and anneal to room temperature in the annealing furnace after keeping it in the muffle furnace for 13 hours at 420°C.
[0099] S21: washing and drying the sample treated in step S13 with deionized wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com