Composition for injectable solution
A composition and medicament technology, applied in the field of injection compositions, can solve problems such as poor solubility, strong irritation of painful bladder, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1: Production example of pirarubicin preparation
[0038] use raw materials
[0039] ・Pirarubicin hydrochloride (also referred to as THP) was prepared by adding hydrochloric acid to pirarubicin (manufactured by Japan Microbio Pharma Co., Ltd.).
[0040] ・With regard to maltose, sucrose and lactose, the products marketed as Japanese Pharmacopoeia products were used as they were.
[0041] ・For trehalose, trehalose produced by Hayashibara Biochemical Research Institute was used as it was.
[0042] 1-1: Lactose 250mg THP 10mg titer The volume of the drug solution (also called the filling amount of the drug solution) at the time of freeze-drying Preparation method of 3mL preparation
[0043]Dissolve 100 mg of pirarubicin hydrochloride (titer) and 2.5 g of lactose in water, adjust the pH to 6 with sodium hydroxide, and after making the total amount to 30 mL, perform sterile filtration and fill each vial with 3 mL Put into a glass vial with a capacity of 15 mL, free...
example 2
[0054] Example 2: Dissolution test of freeze-dried products in physiological saline
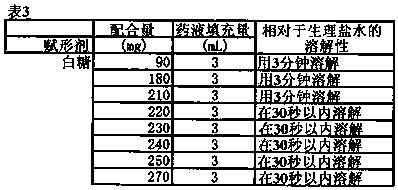
[0055] 10 mL of physiological saline was added to the freeze-dried product obtained as in Example 1, shaken, and the time until the solid matter in the vial was completely measured was measured. The results are shown in Tables 1 to 5 below. The types and amounts of additives contained in 10 mg of pirarubicin hydrochloride constituting the freeze-dried product were used in the amounts shown in the table, respectively.
[0056] [Table 1]
[0057]
[0058] [Table 2]
[0059]
[0060] [table 3]
[0061]
[0062] [Table 4]
[0063]
[0064] [table 5]
[0065]
[0066] In all the test products prepared under the respective conditions in Tables 1 to 5 above, freeze-dried products were obtained without any problem.
example 3
[0067] Example 3: Stability test of freeze-dried products under severe conditions
[0068] Injections (containing 270 mg of maltose) manufactured in freeze-dried products manufactured as described in Example 1 were stored at 60°C and 40°C, and the residual ratio of pirarubicin during the storage periods shown in the table was measured. In addition, as a control substance, the residual rate was measured similarly using the conventional formulation containing 90 mg of lactose per 10 mg of THP. The results are shown in Table 6.
[0069] [Table 6]
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com