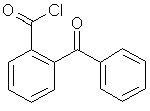
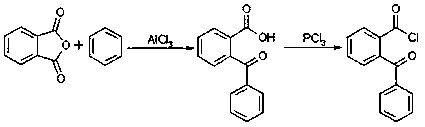
Synthetic method for high-purity 2-(benzoyl) benzoyl chloride
A technology of o-benzoylbenzoic acid and benzoyl chloride is applied in the synthesis field of high-purity 2-benzoyl chloride, can solve the problems of poor production process safety, high requirements on reaction equipment, low product quality, etc. The effect of high product yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 132g of thionyl chloride and 226g of o-benzoylbenzoic acid into a 1000ml four-necked reaction flask, stir evenly, then put in 0.68g of benzyltriethylammonium chloride, start to heat up to 50°C, and keep it warm for 4.5h Finally, TLC tracking shows that the raw material point disappears, the reaction is over, lower to normal temperature, add 340g of chloral, 11.3g of concentrated hydrochloric acid and 0.25g of sulfamic acid, start to heat up, keep warm for 2h after reaching 90°C, TLC tracking shows that the raw material point disappears, the reaction is over, lower to normal temperature, drop 5% liquid caustic soda to adjust the pH of the system, wait until the pH value is about 7, let stand, and separate the water layer. Start rectification to recover chloral (97.5-99°C), the recovery rate is 90%, after the recovery is completed, continue to raise the temperature to recover the middle distillate, the middle distillate includes chloral, water and a small amount of fin...
Embodiment 2
[0026] Put 132g of thionyl chloride and 198g of o-benzoylbenzoic acid into a 1000ml four-necked reaction flask, stir evenly, put in 0.68g of benzyltriethylammonium chloride, start to heat up to 70°C, and keep it warm for 2 hours , TLC tracking shows that the raw material point disappears, the reaction is over, lower to normal temperature, add 198g of chloral, 10g of concentrated hydrochloric acid and 0.20g of sulfamic acid, start to heat up, keep warm for 1h after reaching 105°C, TLC tracking shows that the raw material point disappears , the reaction is completed, lowered to normal temperature, and 5% liquid caustic soda is added dropwise to adjust the pH of the system. When the pH value is about 7, let stand and separate the water layer. Start rectification to recover chloral (97.2-102°C), the recovery rate is 92%. After the recovery is completed, continue to raise the temperature to recover the middle distillate, which includes chloral, water and a small amount of finished p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com