Lithium cobalt phosphate-coated lithium-rich positive electrode material and preparation method thereof
A lithium-rich cathode material, lithium cobalt phosphate technology, applied in battery electrodes, electrical components, electrochemical generators, etc., can solve the problems of poor cycle performance of lithium-rich cathode materials, low initial coulombic efficiency of lithium-rich cathode materials, etc., to achieve The effect of improving the stability of the surface structure, the preparation method is simple, and the cycle performance is improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
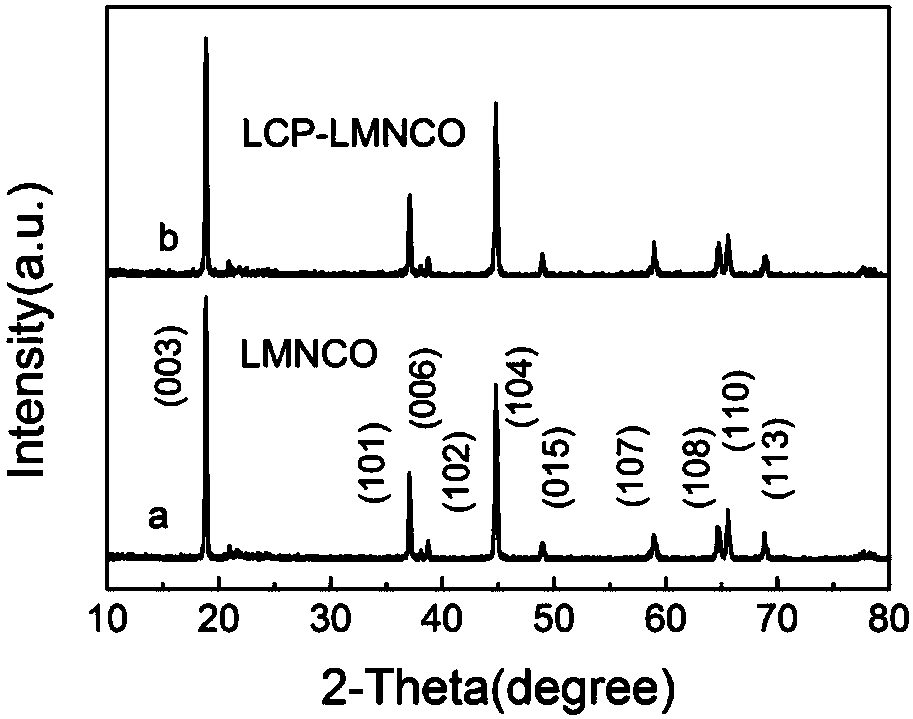
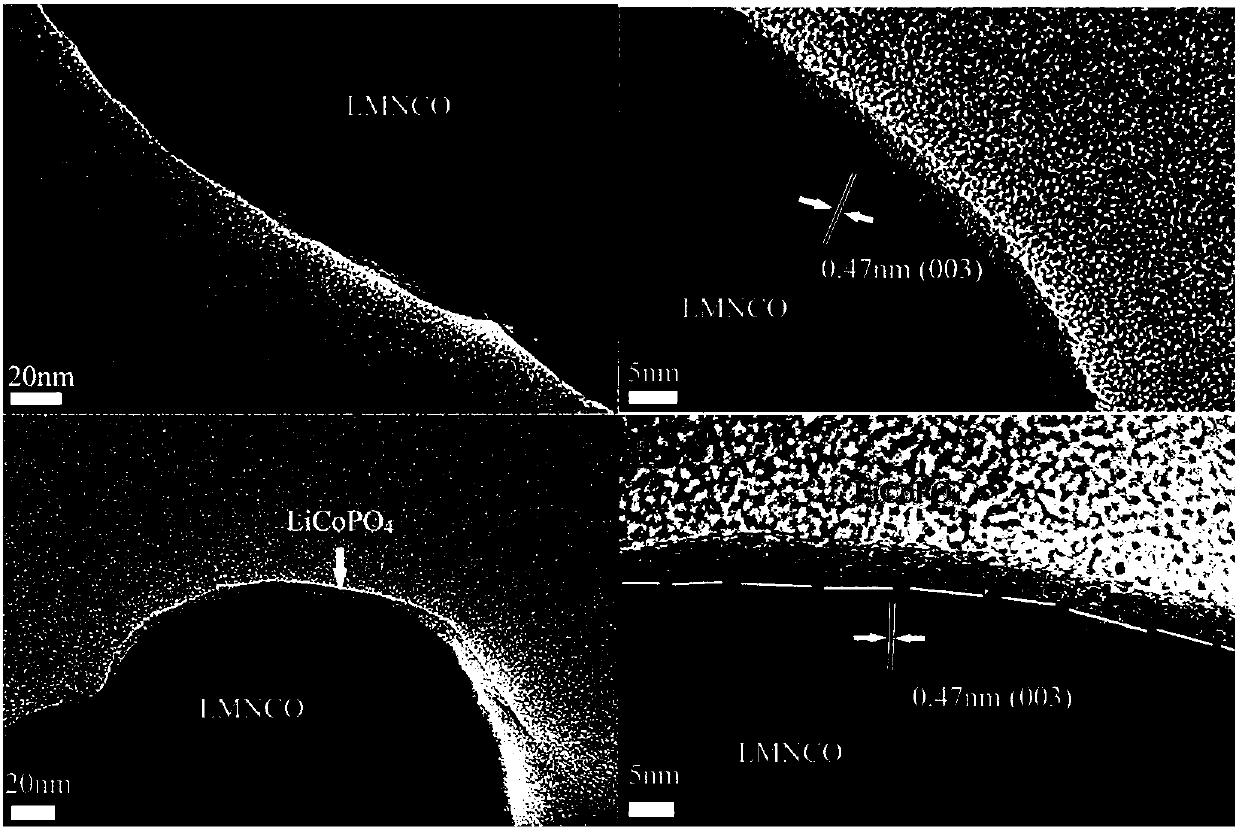
Image
Examples
Embodiment 1
[0026] (1)Li 1.2 Mn 0.54 Ni 0.13 Co 0.13 O 2 Preparation
[0027] Measure LiCOOCH according to the molar ratio Li:Mn:Ni:Co is 1.32:0.54:0.13:0.13 3 ·2H 2 O, Mn(COOCH 3 ) 2 , Ni(COOCH 3 ) 2 ·4H 2 O, Co(COOCH 3 ) 2 ·4H 2 O, dissolved in 100 mL of deionized water to obtain a mixed salt solution; then a certain amount of citric acid (citric acid: metal ion = 4:1 molar ratio) was dissolved in 50 mL of deionized water and stirred evenly; slowly added the citric acid aqueous solution to In the mixed salt solution, heat to 80°C and magnetically stir for 4 hours to form a pink transparent gel. The gel is transferred to a blast drying oven at 80°C for 12 hours and pre-burned in a box furnace under an air atmosphere of 480°C After 5 hours, take it out and grind it, and then move it into a muffle furnace for calcination at 900°C for 12 hours. The heating rate of the calcination stage is 4°C / min, and Li can be obtained. 1.2 Mn 0.54 Ni 0.13 Co 0.13 O 2 (LMNCO) powder.
[0028] (2)LiCoPO 4 Coated...
Embodiment 2
[0033] (1)Li 1.2 Mn 0.54 Ni 0.13 Co 0.13 O 2 Preparation
[0034] Measure LiCOOCH according to the molar ratio Li:Mn:Ni:Co is 1.32:0.54:0.13:0.13 3 ·2H 2 O, Mn(COOCH 3 ) 2 , Ni(COOCH 3 ) 2 ·4H 2 O, Co(COOCH 3 ) 2 ·4H 2 O, dissolved in 100 mL of deionized water to obtain a mixed salt solution; then a certain amount of citric acid (citric acid: metal ion = 4:1 molar ratio) was dissolved in 50 mL of deionized water and stirred evenly; slowly added the citric acid aqueous solution to The mixed salt solution is heated to 80°C and magnetically stirred at a speed of 180r / min for 4 hours to form a pink transparent gel. Transfer the gel to a blast drying oven at 80°C for 12 hours, pre-burn in a box furnace at 480°C for 5 hours, take it out and grind, and then move it into a muffle furnace at 900°C for 12 hours. The heating rate during the calcination stage At 4℃ / min, Li can be obtained 1.2 Mn 0.54 Ni 0.13 Co 0.13 O 2 (LMNCO) powder.
[0035] (2)LiCoPO 4 Coated Li 1.2 Mn 0.54 Ni 0.13 Co 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com